[English] 日本語
 Yorodumi
Yorodumi- PDB-3k3l: Crystal structure of Siderocalin (NGAL, Lipocalin 2) complexed wi... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 3k3l | ||||||
|---|---|---|---|---|---|---|---|
| Title | Crystal structure of Siderocalin (NGAL, Lipocalin 2) complexed with apo Enterobactin | ||||||
 Components Components | Neutrophil gelatinase-associated lipocalin | ||||||
 Keywords Keywords | TRANSPORT PROTEIN / NGAL / p25 / 25 kDa alpha-2-microglobulin-related subunit of MMP-9 / Lipocalin-2 / Oncogene 24p3 / Disulfide bond / Glycoprotein / Secreted / Siderocalin / beta-barrel / siderophore / enterobactin | ||||||
| Function / homology |  Function and homology information Function and homology informationpositive regulation of hippocampal neuron apoptotic process / positive regulation of iron ion import across plasma membrane / positive regulation of endothelial tube morphogenesis / negative regulation of hippocampal neuron apoptotic process / positive regulation of cell projection organization / Metal sequestration by antimicrobial proteins / siderophore transport / response to kainic acid / response to mycotoxin / response to blue light ...positive regulation of hippocampal neuron apoptotic process / positive regulation of iron ion import across plasma membrane / positive regulation of endothelial tube morphogenesis / negative regulation of hippocampal neuron apoptotic process / positive regulation of cell projection organization / Metal sequestration by antimicrobial proteins / siderophore transport / response to kainic acid / response to mycotoxin / response to blue light / cellular response to increased oxygen levels / response to fructose / cellular response to X-ray / short-term memory / cellular response to interleukin-6 / iron ion sequestering activity / enterobactin binding / response to herbicide / response to iron(II) ion / positive regulation of reactive oxygen species biosynthetic process / cellular response to interleukin-1 / long-term memory / cellular response to nutrient levels / extrinsic apoptotic signaling pathway in absence of ligand / positive regulation of endothelial cell migration / acute-phase response / Iron uptake and transport / cellular response to nerve growth factor stimulus / response to virus / specific granule lumen / cellular response to hydrogen peroxide / cellular response to amyloid-beta / cellular response to tumor necrosis factor / positive regulation of cold-induced thermogenesis / cellular response to lipopolysaccharide / protease binding / Interleukin-4 and Interleukin-13 signaling / cellular response to hypoxia / defense response to bacterium / iron ion binding / response to xenobiotic stimulus / innate immune response / Neutrophil degranulation / positive regulation of gene expression / extracellular space / extracellular exosome / extracellular region / identical protein binding Similarity search - Function | ||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / Used previously-determined structure / Resolution: 2.62 Å SYNCHROTRON / Used previously-determined structure / Resolution: 2.62 Å | ||||||
 Authors Authors | Clifton, M.C. | ||||||
 Citation Citation |  Journal: To be Published Journal: To be PublishedTitle: Parsing the functional specificity of Siderocalin / Lipocalin 2 / NGAL for siderophores and related small-molecule ligands Authors: Clifton, M.C. / Rupert, P.B. / Hoette, T.M. / Raymond, K.N. / Abergel, R.J. / Strong, R.K. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  3k3l.cif.gz 3k3l.cif.gz | 113.9 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb3k3l.ent.gz pdb3k3l.ent.gz | 85.8 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  3k3l.json.gz 3k3l.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/k3/3k3l https://data.pdbj.org/pub/pdb/validation_reports/k3/3k3l ftp://data.pdbj.org/pub/pdb/validation_reports/k3/3k3l ftp://data.pdbj.org/pub/pdb/validation_reports/k3/3k3l | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  3cmpC  3hwdC  3hweC  3hwfC  3hwgC  3i0aC  3tf6C  3tnyC  3tzsC  3ioa C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| 2 | 
| ||||||||
| 3 | 
| ||||||||
| Unit cell |
|
- Components
Components
-Protein , 1 types, 3 molecules ABC
| #1: Protein | Mass: 20556.438 Da / Num. of mol.: 3 / Mutation: C87S Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: HNL, LCN2, NGAL / Plasmid: pGEX-4T3 / Production host: Homo sapiens (human) / Gene: HNL, LCN2, NGAL / Plasmid: pGEX-4T3 / Production host:  |
|---|
-Non-polymers , 8 types, 131 molecules 




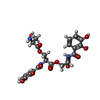









| #2: Chemical | ChemComp-DBS / | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| #3: Chemical | | #4: Chemical | ChemComp-GOL / #5: Chemical | #6: Chemical | ChemComp-SO4 / | #7: Chemical | ChemComp-MCK / | #8: Chemical | ChemComp-CL / | #9: Water | ChemComp-HOH / | |
-Details
| Has protein modification | Y |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 3.15 Å3/Da / Density % sol: 61 % |
|---|---|
| Crystal grow | Temperature: 291 K / Method: vapor diffusion, hanging drop / pH: 4.5 Details: 1.3M Ammonium sulfate, 0.2M Lithium sulfate, 50mM sodium chloride, 0.1M sodium acetate, pH 4.5, VAPOR DIFFUSION, HANGING DROP, temperature 291K |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  ALS ALS  / Beamline: 5.0.1 / Beamline: 5.0.1 |
| Detector | Type: ADSC QUANTUM 210 / Detector: CCD |
| Radiation | Monochromator: Single crystal, cylindrically bent, Si(220) / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Relative weight: 1 |
| Reflection | Resolution: 2.62→50 Å / Num. all: 44579 / Num. obs: 23965 / % possible obs: 100 % / Redundancy: 12.9 % / Rmerge(I) obs: 0.057 / Net I/σ(I): 50.3 |
| Reflection shell | Resolution: 2.62→2.7 Å / Redundancy: 13.2 % / Rmerge(I) obs: 0.41 / Mean I/σ(I) obs: 7.51 / Num. unique all: 2347 / % possible all: 100 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure: Used previously-determined structure Resolution: 2.62→41.25 Å / Cor.coef. Fo:Fc: 0.893 / Cor.coef. Fo:Fc free: 0.854 / SU B: 11.023 / SU ML: 0.243 / Cross valid method: THROUGHOUT / ESU R: 0.575 / ESU R Free: 0.355 / Stereochemistry target values: MAXIMUM LIKELIHOOD / Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.2 Å / Solvent model: MASK | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 36.449 Å2
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.62→41.25 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 2.623→2.691 Å / Total num. of bins used: 20
|
 Movie
Movie Controller
Controller









 PDBj
PDBj








