+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-32046 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of human KCNQ4-ML213 complex in digitonin | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | KCNQ4 / ML213 / cryo-EM / digitonin / MEMBRANE PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationtransporter inhibitor activity / Voltage gated Potassium channels / : / type 3 metabotropic glutamate receptor binding / Sensory processing of sound by outer hair cells of the cochlea / Sensory processing of sound by inner hair cells of the cochlea / inner ear morphogenesis / response to corticosterone / negative regulation of high voltage-gated calcium channel activity / negative regulation of calcium ion export across plasma membrane ...transporter inhibitor activity / Voltage gated Potassium channels / : / type 3 metabotropic glutamate receptor binding / Sensory processing of sound by outer hair cells of the cochlea / Sensory processing of sound by inner hair cells of the cochlea / inner ear morphogenesis / response to corticosterone / negative regulation of high voltage-gated calcium channel activity / negative regulation of calcium ion export across plasma membrane / regulation of cardiac muscle cell action potential / presynaptic endocytosis / nitric-oxide synthase binding / regulation of synaptic vesicle exocytosis / regulation of cell communication by electrical coupling involved in cardiac conduction / calcineurin-mediated signaling / adenylate cyclase binding / protein phosphatase activator activity / carbohydrate transmembrane transporter activity / maltose binding / voltage-gated potassium channel activity / maltose transport / regulation of synaptic vesicle endocytosis / maltodextrin transmembrane transport / detection of calcium ion / potassium channel activity / regulation of cardiac muscle contraction / postsynaptic cytosol / catalytic complex / regulation of cardiac muscle contraction by regulation of the release of sequestered calcium ion / phosphatidylinositol 3-kinase binding / presynaptic cytosol / ATP-binding cassette (ABC) transporter complex, substrate-binding subunit-containing / regulation of release of sequestered calcium ion into cytosol by sarcoplasmic reticulum / titin binding / regulation of calcium-mediated signaling / sperm midpiece / voltage-gated potassium channel complex / potassium ion transmembrane transport / calcium channel complex / substantia nigra development / regulation of heart rate / basal plasma membrane / calyx of Held / response to amphetamine / adenylate cyclase activator activity / nitric-oxide synthase regulator activity / sarcomere / protein serine/threonine kinase activator activity / regulation of cytokinesis / spindle microtubule / calcium channel regulator activity / sensory perception of sound / response to calcium ion / potassium ion transport / mitochondrial membrane / Schaffer collateral - CA1 synapse / G2/M transition of mitotic cell cycle / long-term synaptic potentiation / spindle pole / calcium-dependent protein binding / synaptic vesicle membrane / myelin sheath / outer membrane-bounded periplasmic space / growth cone / vesicle / transmembrane transporter binding / G protein-coupled receptor signaling pathway / protein domain specific binding / calcium ion binding / centrosome / protein kinase binding / protein-containing complex / nucleus / plasma membrane / cytoplasm Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) / Homo sapiens (human) /  | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 2.8 Å | |||||||||
 Authors Authors | Xu F / Zheng Y | |||||||||
| Funding support |  China, 1 items China, 1 items
| |||||||||
 Citation Citation |  Journal: Neuron / Year: 2022 Journal: Neuron / Year: 2022Title: Structural insights into the lipid and ligand regulation of a human neuronal KCNQ channel. Authors: You Zheng / Heng Liu / Yuxin Chen / Shaowei Dong / Fang Wang / Shengyi Wang / Geng-Lin Li / Yilai Shu / Fei Xu /  Abstract: The KCNQ family (KCNQ1-KCNQ5) of voltage-gated potassium channels plays critical roles in many physiological and pathological processes. It is known that the channel opening of all KCNQs relies on ...The KCNQ family (KCNQ1-KCNQ5) of voltage-gated potassium channels plays critical roles in many physiological and pathological processes. It is known that the channel opening of all KCNQs relies on the signaling lipid molecule phosphatidylinositol 4,5-bisphosphate (PIP2). However, the molecular mechanism of PIP2 in modulating the opening of the four neuronal KCNQ channels (KCNQ2-KCNQ5), which are essential for regulating neuronal excitability, remains largely elusive. Here, we report the cryoelectron microscopy (cryo-EM) structures of human KCNQ4 determined in complex with the activator ML213 in the absence or presence of PIP2. Two PIP2 molecules are identified in the open-state structure of KCNQ4, which act as a bridge to couple the voltage-sensing domain (VSD) and pore domain (PD) of KCNQ4 leading to the channel opening. Our findings reveal the binding sites and activation mechanisms of ML213 and PIP2 for neuronal KCNQ channels, providing a framework for therapeutic intervention targeting on these important channels. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_32046.map.gz emd_32046.map.gz | 62.2 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-32046-v30.xml emd-32046-v30.xml emd-32046.xml emd-32046.xml | 12.4 KB 12.4 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_32046.png emd_32046.png | 75.8 KB | ||
| Filedesc metadata |  emd-32046.cif.gz emd-32046.cif.gz | 6.1 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-32046 http://ftp.pdbj.org/pub/emdb/structures/EMD-32046 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-32046 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-32046 | HTTPS FTP |
-Validation report
| Summary document |  emd_32046_validation.pdf.gz emd_32046_validation.pdf.gz | 481.9 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_32046_full_validation.pdf.gz emd_32046_full_validation.pdf.gz | 481.5 KB | Display | |
| Data in XML |  emd_32046_validation.xml.gz emd_32046_validation.xml.gz | 6.6 KB | Display | |
| Data in CIF |  emd_32046_validation.cif.gz emd_32046_validation.cif.gz | 7.5 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-32046 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-32046 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-32046 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-32046 | HTTPS FTP |
-Related structure data
| Related structure data |  7vnrMC  7vnpC  7vnqC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_32046.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_32046.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.82 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : KCNQ4-ML213 complex in digitonin
| Entire | Name: KCNQ4-ML213 complex in digitonin |
|---|---|
| Components |
|
-Supramolecule #1: KCNQ4-ML213 complex in digitonin
| Supramolecule | Name: KCNQ4-ML213 complex in digitonin / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#2 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Potassium voltage-gated channel subfamily KQT member 4,Maltodextr...
| Macromolecule | Name: Potassium voltage-gated channel subfamily KQT member 4,Maltodextrin-binding protein type: protein_or_peptide / ID: 1 Details: The fusion protein of Potassium voltage-gated channel subfamily KQT member 4, linker, and Maltodextrin-binding protein Number of copies: 4 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 116.541383 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MDYKDDDDKA EAPPRRLGLG PPPGDAPRAE LVALTAVQSE QGEAGGGGSP RRLGLLGSPL PPGAPLPGPG SGSGSACGQR SSAAHKRYR RLQNWVYNVL ERPRGWAFVY HVFIFLLVFS CLVLSVLSTI QEHQELANEC LLILEFVMIV VFGLEYIVRV W SAGCCCRY ...String: MDYKDDDDKA EAPPRRLGLG PPPGDAPRAE LVALTAVQSE QGEAGGGGSP RRLGLLGSPL PPGAPLPGPG SGSGSACGQR SSAAHKRYR RLQNWVYNVL ERPRGWAFVY HVFIFLLVFS CLVLSVLSTI QEHQELANEC LLILEFVMIV VFGLEYIVRV W SAGCCCRY RGWQGRFRFA RKPFCVIDFI VFVASVAVIA AGTQGNIFAT SALRSMRFLQ ILRMVRMDRR GGTWKLLGSV VY AHSKELI TAWYIGFLVL IFASFLVYLA EKDANSDFSS YADSLWWGTI TLTTIGYGDK TPHTWLGRVL AAGFALLGIS FFA LPAGIL GSGFALKVQE QHRQKHFEKR RMPAANLIQA AWRLYSTDMS RAYLTATWYY YDSILPSFRE LALLFEHVQR ARNG GLRPL EVRRAPVPDG APSRYPPVAT CHRPGSTSFC PGESSRMGIK DRIRMGSSQR RTGPSKQHLA PPTMPTSPSS EQVGE ATSP TKVQKSWSFN DRTRFRASLR LKPRTSAEDA PSEEVAEEKS YQCELTVDDI MPAVKTVIRS IRILKFLVAK RKFKET LRP YDVKDVIEQY SAGHLDMLGR IKSLQTRVDQ IVGRGPGDRK AREKGDKGPS DAEVVDEISM MGRVVKVEKQ VQSIEHK LD LLLGFYSRCL RSGTSALEVL FQGPMAKIEE GKLVIWINGD KGYNGLAEVG KKFEKDTGIK VTVEHPDKLE EKFPQVAA T GDGPDIIFWA HDRFGGYAQS GLLAEITPDK AFQDKLYPFT WDAVRYNGKL IAYPIAVEAL SLIYNKDLLP NPPKTWEEI PALDKELKAK GKSALMFNLQ EPYFTWPLIA ADGGYAFKYE NGKYDIKDVG VDNAGAKAGL TFLVDLIKNK HMNADTDYSI AEAAFNKGE TAMTINGPWA WSNIDTSKVN YGVTVLPTFK GQPSKPFVGV LSAGINAASP NKELAKEFLE NYLLTDEGLE A VNKDKPLG AVALKSYEEE LAKDPRIAAT MENAQKGEIM PNIPQMSAFW YAVRTAVINA ASGRQTVDEA LKDAQTNAAA EH HHHHHHH HH UniProtKB: Potassium voltage-gated channel subfamily KQT member 4, Maltodextrin-binding protein |
-Macromolecule #2: Calmodulin-3
| Macromolecule | Name: Calmodulin-3 / type: protein_or_peptide / ID: 2 / Number of copies: 4 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 16.852545 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MADQLTEEQI AEFKEAFSLF DKDGDGTITT KELGTVMRSL GQNPTEAELQ DMINEVDADG NGTIDFPEFL TMMARKMKDT DSEEEIREA FRVFDKDGNG YISAAELRHV MTNLGEKLTD EEVDEMIREA DIDGDGQVNY EEFVQMMTAK UniProtKB: Calmodulin-3 |
-Macromolecule #3: (1S,2S,4R)-N-(2,4,6-trimethylphenyl)bicyclo[2.2.1]heptane-2-carboxamid
| Macromolecule | Name: (1S,2S,4R)-N-(2,4,6-trimethylphenyl)bicyclo[2.2.1]heptane-2-carboxamid type: ligand / ID: 3 / Number of copies: 4 / Formula: 7YV |
|---|---|
| Molecular weight | Theoretical: 257.371 Da |
| Chemical component information | 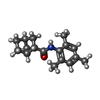 ChemComp-7YV: |
-Macromolecule #4: POTASSIUM ION
| Macromolecule | Name: POTASSIUM ION / type: ligand / ID: 4 / Number of copies: 3 / Formula: K |
|---|---|
| Molecular weight | Theoretical: 39.098 Da |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 4.8 mg/mL |
|---|---|
| Buffer | pH: 7.4 |
| Grid | Model: Quantifoil R1.2/1.3 / Material: GOLD / Mesh: 300 / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 45 sec. |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 16.8 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: DARK FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: NONE |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 2.8 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 126225 |
| Initial angle assignment | Type: MAXIMUM LIKELIHOOD |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
 Movie
Movie Controller
Controller

















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)





















