[English] 日本語
 Yorodumi
Yorodumi- EMDB-23436: Oxyntomodulin-bound Glucagon-Like Peptide-1 (GLP-1) Receptor in c... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-23436 | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Oxyntomodulin-bound Glucagon-Like Peptide-1 (GLP-1) Receptor in complex with Gs protein | ||||||||||||||||||
 Map data Map data | postprocess map | ||||||||||||||||||
 Sample Sample |
| ||||||||||||||||||
 Keywords Keywords | Glucagon-Like Peptide-1 (GLP-1) Receptor / Oxyntomodulin / MEMBRANE PROTEIN | ||||||||||||||||||
| Function / homology |  Function and homology information Function and homology informationGlucagon signaling in metabolic regulation / Glucagon-like Peptide-1 (GLP1) regulates insulin secretion / G alpha (s) signalling events / Glucagon-type ligand receptors / Synthesis, secretion, and inactivation of Glucagon-like Peptide-1 (GLP-1) / G alpha (q) signalling events / Synthesis, secretion, and deacylation of Ghrelin / glucagon receptor binding / glucagon-like peptide 1 receptor activity / glucagon receptor activity ...Glucagon signaling in metabolic regulation / Glucagon-like Peptide-1 (GLP1) regulates insulin secretion / G alpha (s) signalling events / Glucagon-type ligand receptors / Synthesis, secretion, and inactivation of Glucagon-like Peptide-1 (GLP-1) / G alpha (q) signalling events / Synthesis, secretion, and deacylation of Ghrelin / glucagon receptor binding / glucagon-like peptide 1 receptor activity / glucagon receptor activity / hormone secretion / positive regulation of blood pressure / post-translational protein targeting to membrane, translocation / regulation of heart contraction / response to psychosocial stress / positive regulation of insulin secretion involved in cellular response to glucose stimulus / PKA activation in glucagon signalling / peptide hormone binding / hair follicle placode formation / developmental growth / D1 dopamine receptor binding / intracellular transport / renal water homeostasis / Hedgehog 'off' state / adenylate cyclase-activating adrenergic receptor signaling pathway / activation of adenylate cyclase activity / cellular response to glucagon stimulus / protein kinase A signaling / negative regulation of blood pressure / adenylate cyclase activator activity / regulation of insulin secretion / cAMP-mediated signaling / trans-Golgi network membrane / response to activity / negative regulation of inflammatory response to antigenic stimulus / bone development / G-protein beta/gamma-subunit complex binding / adenylate cyclase-activating G protein-coupled receptor signaling pathway / Olfactory Signaling Pathway / Activation of the phototransduction cascade / adenylate cyclase-modulating G protein-coupled receptor signaling pathway / hormone activity / G protein activity / G beta:gamma signalling through PLC beta / Presynaptic function of Kainate receptors / Thromboxane signalling through TP receptor / G protein-coupled acetylcholine receptor signaling pathway / G-protein activation / platelet aggregation / Activation of G protein gated Potassium channels / Inhibition of voltage gated Ca2+ channels via Gbeta/gamma subunits / Prostacyclin signalling through prostacyclin receptor / Glucagon signaling in metabolic regulation / G beta:gamma signalling through CDC42 / cognition / G beta:gamma signalling through BTK / ADP signalling through P2Y purinoceptor 12 / Sensory perception of sweet, bitter, and umami (glutamate) taste / Synthesis, secretion, and inactivation of Glucagon-like Peptide-1 (GLP-1) / photoreceptor disc membrane / Glucagon-type ligand receptors / Adrenaline,noradrenaline inhibits insulin secretion / Vasopressin regulates renal water homeostasis via Aquaporins / G alpha (z) signalling events / Glucagon-like Peptide-1 (GLP1) regulates insulin secretion / cellular response to catecholamine stimulus / ADORA2B mediated anti-inflammatory cytokines production / sensory perception of taste / ADP signalling through P2Y purinoceptor 1 / G beta:gamma signalling through PI3Kgamma / adenylate cyclase-activating dopamine receptor signaling pathway / Cooperation of PDCL (PhLP1) and TRiC/CCT in G-protein beta folding / GPER1 signaling / cellular response to prostaglandin E stimulus / Inactivation, recovery and regulation of the phototransduction cascade / G-protein beta-subunit binding / transmembrane signaling receptor activity / heterotrimeric G-protein complex / sensory perception of smell / G alpha (12/13) signalling events / extracellular vesicle / signaling receptor complex adaptor activity / Thrombin signalling through proteinase activated receptors (PARs) / GTPase binding / glucose homeostasis / retina development in camera-type eye / positive regulation of cold-induced thermogenesis / Ca2+ pathway / phospholipase C-activating G protein-coupled receptor signaling pathway / positive regulation of cytosolic calcium ion concentration / G alpha (i) signalling events / fibroblast proliferation / Hydrolases; Acting on acid anhydrides; Acting on GTP to facilitate cellular and subcellular movement / G alpha (s) signalling events / G alpha (q) signalling events / Ras protein signal transduction / cell population proliferation / Extra-nuclear estrogen signaling / learning or memory / cell surface receptor signaling pathway Similarity search - Function | ||||||||||||||||||
| Biological species |  Homo sapiens (human) / Homo sapiens (human) /   | ||||||||||||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.3 Å | ||||||||||||||||||
 Authors Authors | Wootten D / Sexton PM | ||||||||||||||||||
| Funding support |  Australia, Australia,  Japan, 5 items Japan, 5 items
| ||||||||||||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2022 Journal: Nat Commun / Year: 2022Title: Dynamics of GLP-1R peptide agonist engagement are correlated with kinetics of G protein activation. Authors: Giuseppe Deganutti / Yi-Lynn Liang / Xin Zhang / Maryam Khoshouei / Lachlan Clydesdale / Matthew J Belousoff / Hari Venugopal / Tin T Truong / Alisa Glukhova / Andrew N Keller / Karen J ...Authors: Giuseppe Deganutti / Yi-Lynn Liang / Xin Zhang / Maryam Khoshouei / Lachlan Clydesdale / Matthew J Belousoff / Hari Venugopal / Tin T Truong / Alisa Glukhova / Andrew N Keller / Karen J Gregory / Katie Leach / Arthur Christopoulos / Radostin Danev / Christopher A Reynolds / Peishen Zhao / Patrick M Sexton / Denise Wootten /       Abstract: The glucagon-like peptide-1 receptor (GLP-1R) has broad physiological roles and is a validated target for treatment of metabolic disorders. Despite recent advances in GLP-1R structure elucidation, ...The glucagon-like peptide-1 receptor (GLP-1R) has broad physiological roles and is a validated target for treatment of metabolic disorders. Despite recent advances in GLP-1R structure elucidation, detailed mechanistic understanding of how different peptides generate profound differences in G protein-mediated signalling is still lacking. Here we combine cryo-electron microscopy, molecular dynamics simulations, receptor mutagenesis and pharmacological assays, to interrogate the mechanism and consequences of GLP-1R binding to four peptide agonists; glucagon-like peptide-1, oxyntomodulin, exendin-4 and exendin-P5. These data reveal that distinctions in peptide N-terminal interactions and dynamics with the GLP-1R transmembrane domain are reciprocally associated with differences in the allosteric coupling to G proteins. In particular, transient interactions with residues at the base of the binding cavity correlate with enhanced kinetics for G protein activation, providing a rationale for differences in G protein-mediated signalling efficacy from distinct agonists. | ||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_23436.map.gz emd_23436.map.gz | 3.6 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-23436-v30.xml emd-23436-v30.xml emd-23436.xml emd-23436.xml | 21.8 KB 21.8 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_23436_fsc.xml emd_23436_fsc.xml | 6.5 KB | Display |  FSC data file FSC data file |
| Images |  emd_23436.png emd_23436.png | 84.6 KB | ||
| Masks |  emd_23436_msk_1.map emd_23436_msk_1.map | 22.2 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-23436.cif.gz emd-23436.cif.gz | 6.8 KB | ||
| Others |  emd_23436_half_map_1.map.gz emd_23436_half_map_1.map.gz emd_23436_half_map_2.map.gz emd_23436_half_map_2.map.gz | 17 MB 17 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-23436 http://ftp.pdbj.org/pub/emdb/structures/EMD-23436 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-23436 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-23436 | HTTPS FTP |
-Validation report
| Summary document |  emd_23436_validation.pdf.gz emd_23436_validation.pdf.gz | 666.5 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_23436_full_validation.pdf.gz emd_23436_full_validation.pdf.gz | 666.1 KB | Display | |
| Data in XML |  emd_23436_validation.xml.gz emd_23436_validation.xml.gz | 12 KB | Display | |
| Data in CIF |  emd_23436_validation.cif.gz emd_23436_validation.cif.gz | 16.7 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-23436 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-23436 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-23436 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-23436 | HTTPS FTP |
-Related structure data
| Related structure data |  7llyMC  7lllC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_23436.map.gz / Format: CCP4 / Size: 22.2 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_23436.map.gz / Format: CCP4 / Size: 22.2 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | postprocess map | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.06 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
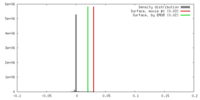
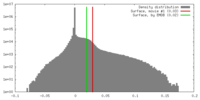
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Mask #1
| File |  emd_23436_msk_1.map emd_23436_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
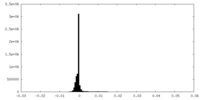
| Density Histograms |
-Half map: half map 1
| File | emd_23436_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half map 1 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: half map 2
| File | emd_23436_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half map 2 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Oxyntomodulin-GLP-1R-Gs complex
| Entire | Name: Oxyntomodulin-GLP-1R-Gs complex |
|---|---|
| Components |
|
-Supramolecule #1: Oxyntomodulin-GLP-1R-Gs complex
| Supramolecule | Name: Oxyntomodulin-GLP-1R-Gs complex / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Guanine nucleotide-binding protein G(s) subunit alpha isoforms short
| Macromolecule | Name: Guanine nucleotide-binding protein G(s) subunit alpha isoforms short type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 45.683434 KDa |
| Recombinant expression | Organism:  Trichoplusia ni (cabbage looper) Trichoplusia ni (cabbage looper) |
| Sequence | String: MGCLGNSKTE DQRNEEKAQR EANKKIEKQL QKDKQVYRAT HRLLLLGAGE SGKNTIVKQM RILHVNGFNG EGGEEDPQAA RSNSDGEKA TKVQDIKNNL KEAIETIVAA MSNLVPPVEL ANPENQFRVD YILSVMNVPD FDFPPEFYEH AKALWEDEGV R ACYERSNE ...String: MGCLGNSKTE DQRNEEKAQR EANKKIEKQL QKDKQVYRAT HRLLLLGAGE SGKNTIVKQM RILHVNGFNG EGGEEDPQAA RSNSDGEKA TKVQDIKNNL KEAIETIVAA MSNLVPPVEL ANPENQFRVD YILSVMNVPD FDFPPEFYEH AKALWEDEGV R ACYERSNE YQLIDCAQYF LDKIDVIKQA DYVPSDQDLL RCRVLTSGIF ETKFQVDKVN FHMFDVGAQR DERRKWIQCF ND VTAIIFV VASSSYNMVI REDNQTNRLQ AALKLFDSIW NNKWLRDTSV ILFLNKQDLL AEKVLAGKSK IEDYFPEFAR YTT PEDATP EPGEDPRVTR AKYFIRDEFL RISTASGDGR HYCYPHFTCA VDTENIRRVF NDCRDIIQRM HLRQYELL UniProtKB: Guanine nucleotide-binding protein G(s) subunit alpha isoforms short |
-Macromolecule #2: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1
| Macromolecule | Name: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1 type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 37.413863 KDa |
| Recombinant expression | Organism:  Trichoplusia ni (cabbage looper) Trichoplusia ni (cabbage looper) |
| Sequence | String: QSELDQLRQE AEQLKNQIRD ARKACADATL SQITNNIDPV GRIQMRTRRT LRGHLAKIYA MHWGTDSRLL VSASQDGKLI IWDSYTTNK VHAIPLRSSW VMTCAYAPSG NYVACGGLDN ICSIYNLKTR EGNVRVSREL AGHTGYLSCC RFLDDNQIVT S SGDTTCAL ...String: QSELDQLRQE AEQLKNQIRD ARKACADATL SQITNNIDPV GRIQMRTRRT LRGHLAKIYA MHWGTDSRLL VSASQDGKLI IWDSYTTNK VHAIPLRSSW VMTCAYAPSG NYVACGGLDN ICSIYNLKTR EGNVRVSREL AGHTGYLSCC RFLDDNQIVT S SGDTTCAL WDIETGQQTT TFTGHTGDVM SLSLAPDTRL FVSGACDASA KLWDVREGMC RQTFTGHESD INAICFFPNG NA FATGSDD ATCRLFDLRA DQELMTYSHD NIICGITSVS FSKSGRLLLA GYDDFNCNVW DALKADRAGV LAGHDNRVSC LGV TDDGMA VATGSWDSFL KIWN UniProtKB: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1 |
-Macromolecule #3: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2
| Macromolecule | Name: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2 type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 6.375332 KDa |
| Recombinant expression | Organism:  Trichoplusia ni (cabbage looper) Trichoplusia ni (cabbage looper) |
| Sequence | String: NTASIAQARK LVEQLKMEAN IDRIKVSKAA ADLMAYCEAH AKEDPLLTPV PASENPFR UniProtKB: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2 |
-Macromolecule #4: Nb35
| Macromolecule | Name: Nb35 / type: protein_or_peptide / ID: 4 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 13.885439 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: QVQLQESGGG LVQPGGSLRL SCAASGFTFS NYKMNWVRQA PGKGLEWVSD ISQSGASISY TGSVKGRFTI SRDNAKNTLY LQMNSLKPE DTAVYYCARC PAPFTRDCFD VTSTTYAYRG QGTQVTVSS |
-Macromolecule #5: Pro-glucagon
| Macromolecule | Name: Pro-glucagon / type: protein_or_peptide / ID: 5 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 4.428878 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: HSQGTFTSDY SKYLDSRRAQ DFVQWLMNTK RNKNNIA UniProtKB: Pro-glucagon |
-Macromolecule #6: Glucagon-like peptide 1 receptor
| Macromolecule | Name: Glucagon-like peptide 1 receptor / type: protein_or_peptide / ID: 6 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 56.748418 KDa |
| Recombinant expression | Organism:  Trichoplusia ni (cabbage looper) Trichoplusia ni (cabbage looper) |
| Sequence | String: MKTIIALSYI FCLVFADYKD DDDLEVLFQG PARPQGATVS LWETVQKWRE YRRQCQRSLT EDPPPATDLF CNRTFDEYAC WPDGEPGSF VNVSCPWYLP WASSVPQGHV YRFCTAEGLW LQKDNSSLPW RDLSECEESK RGERSSPEEQ LLFLYIIYTV G YALSFSAL ...String: MKTIIALSYI FCLVFADYKD DDDLEVLFQG PARPQGATVS LWETVQKWRE YRRQCQRSLT EDPPPATDLF CNRTFDEYAC WPDGEPGSF VNVSCPWYLP WASSVPQGHV YRFCTAEGLW LQKDNSSLPW RDLSECEESK RGERSSPEEQ LLFLYIIYTV G YALSFSAL VIASAILLGF RHLHCTRNYI HLNLFASFIL RALSVFIKDA ALKWMYSTAA QQHQWDGLLS YQDSLSCRLV FL LMQYCVA ANYYWLLVEG VYLYTLLAFS VFSEQWIFRL YVSIGWGVPL LFVVPWGIVK YLYEDEGCWT RNSNMNYWLI IRL PILFAI GVNFLIFVRV ICIVVSKLKA NLMCKTDIKC RLAKSTLTLI PLLGTHEVIF AFVMDEHARG TLRFIKLFTE LSFT SFQGL MVAILYCFVN NEVQLEFRKS WERWRLEHLH IQRDSSMKPL KCPTSSLSSG ATAGSSMYTA TCQASCSPAG LEVLF QGPH HHHHHHH UniProtKB: Glucagon-like peptide 1 receptor |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Specialist optics | Phase plate: VOLTA PHASE PLATE Spherical aberration corrector: Non-Cs corrected microscope with a Cs=2.7 mm |
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: COUNTING / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller

































 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)