[English] 日本語
 Yorodumi
Yorodumi- EMDB-22321: Cryo-EM structure of bedaquiline-saturated Mycobacterium smegmati... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-22321 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of bedaquiline-saturated Mycobacterium smegmatis ATP synthase FO region | |||||||||
 Map data Map data | Locally sharpened map. | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Bedaquiline / Sirturo / TMC207 / T207910 / ATP synthase / mycobacteria / tuberculosis / HYDROLASE | |||||||||
| Function / homology |  Function and homology information Function and homology information: / proton motive force-driven plasma membrane ATP synthesis / proton-transporting two-sector ATPase complex, proton-transporting domain / proton-transporting ATPase activity, rotational mechanism / membrane => GO:0016020 / proton-transporting ATP synthase complex / proton-transporting ATP synthase activity, rotational mechanism / hydrolase activity / lipid binding / plasma membrane Similarity search - Function | |||||||||
| Biological species |  Mycolicibacterium smegmatis (bacteria) Mycolicibacterium smegmatis (bacteria) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.4 Å | |||||||||
 Authors Authors | Guo H / Courbon GM | |||||||||
| Funding support |  Canada, 2 items Canada, 2 items
| |||||||||
 Citation Citation |  Journal: Nature / Year: 2021 Journal: Nature / Year: 2021Title: Structure of mycobacterial ATP synthase bound to the tuberculosis drug bedaquiline. Authors: Hui Guo / Gautier M Courbon / Stephanie A Bueler / Juntao Mai / Jun Liu / John L Rubinstein /  Abstract: Tuberculosis-the world's leading cause of death by infectious disease-is increasingly resistant to current first-line antibiotics. The bacterium Mycobacterium tuberculosis (which causes tuberculosis) ...Tuberculosis-the world's leading cause of death by infectious disease-is increasingly resistant to current first-line antibiotics. The bacterium Mycobacterium tuberculosis (which causes tuberculosis) can survive low-energy conditions, allowing infections to remain dormant and decreasing their susceptibility to many antibiotics. Bedaquiline was developed in 2005 from a lead compound identified in a phenotypic screen against Mycobacterium smegmatis. This drug can sterilize even latent M. tuberculosis infections and has become a cornerstone of treatment for multidrug-resistant and extensively drug-resistant tuberculosis. Bedaquiline targets the mycobacterial ATP synthase, which is an essential enzyme in the obligate aerobic Mycobacterium genus, but how it binds the intact enzyme is unknown. Here we determined cryo-electron microscopy structures of M. smegmatis ATP synthase alone and in complex with bedaquiline. The drug-free structure suggests that hook-like extensions from the α-subunits prevent the enzyme from running in reverse, inhibiting ATP hydrolysis and preserving energy in hypoxic conditions. Bedaquiline binding induces large conformational changes in the ATP synthase, creating tight binding pockets at the interface of subunits a and c that explain the potency of this drug as an antibiotic for tuberculosis. #1:  Journal: Biorxiv / Year: 2020 Journal: Biorxiv / Year: 2020Title: Structure of mycobacterial ATP synthase with the TB drug bedaquiline Authors: Guo H / Courbon GM / Bueler SA / Mai J / Liu J / Rubinstein JL | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_22321.map.gz emd_22321.map.gz | 59 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-22321-v30.xml emd-22321-v30.xml emd-22321.xml emd-22321.xml | 33 KB 33 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_22321_fsc.xml emd_22321_fsc.xml | 11.4 KB | Display |  FSC data file FSC data file |
| Images |  emd_22321.png emd_22321.png | 120.5 KB | ||
| Masks |  emd_22321_msk_1.map emd_22321_msk_1.map | 125 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-22321.cif.gz emd-22321.cif.gz | 7.6 KB | ||
| Others |  emd_22321_additional.map.gz emd_22321_additional.map.gz emd_22321_additional_1.map.gz emd_22321_additional_1.map.gz emd_22321_half_map_1.map.gz emd_22321_half_map_1.map.gz emd_22321_half_map_2.map.gz emd_22321_half_map_2.map.gz | 62.4 MB 62.4 MB 116.2 MB 116.2 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-22321 http://ftp.pdbj.org/pub/emdb/structures/EMD-22321 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-22321 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-22321 | HTTPS FTP |
-Related structure data
| Related structure data |  7jgcMC  7jg5C  7jg6C  7jg7C  7jg8C  7jg9C  7jgaC  7jgbC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_22321.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_22321.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Locally sharpened map. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.03 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Mask #1
| File |  emd_22321_msk_1.map emd_22321_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
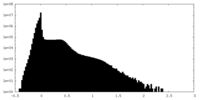
| Density Histograms |
-Additional map: Unsharpened map.
| File | emd_22321_additional.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Unsharpened map. | ||||||||||||
| Projections & Slices |
| ||||||||||||
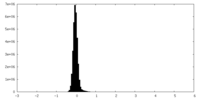
| Density Histograms |
-Additional map: Unsharpened map.
| File | emd_22321_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Unsharpened map. | ||||||||||||
| Projections & Slices |
| ||||||||||||
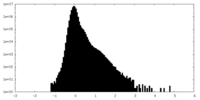
| Density Histograms |
-Half map: Half map 1.
| File | emd_22321_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map 1. | ||||||||||||
| Projections & Slices |
| ||||||||||||
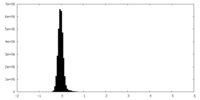
| Density Histograms |
-Half map: Half map 2.
| File | emd_22321_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map 2. | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : FO region of ATP synthase from Mycobacterium smegmatis
| Entire | Name: FO region of ATP synthase from Mycobacterium smegmatis |
|---|---|
| Components |
|
-Supramolecule #1: FO region of ATP synthase from Mycobacterium smegmatis
| Supramolecule | Name: FO region of ATP synthase from Mycobacterium smegmatis type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#4 |
|---|---|
| Source (natural) | Organism:  Mycolicibacterium smegmatis (bacteria) Mycolicibacterium smegmatis (bacteria) |
| Molecular weight | Theoretical: 110 KDa |
-Macromolecule #1: ATP synthase subunit a
| Macromolecule | Name: ATP synthase subunit a / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Mycolicibacterium smegmatis (bacteria) Mycolicibacterium smegmatis (bacteria) |
| Molecular weight | Theoretical: 27.568482 KDa |
| Sequence | String: MLAAEEGGAA IHVGHHTLVF ELFGMTFNGD TILATAVTAV IVIALAFYLR AKVTSTGVPS GVQLFWEALT IQMRQQIEGS IGMKIAPFV LPLSVTIFVF ILISNWLAVL PLQYGGADGA AAELYKAPAS DINFVLALAL FVFVCYHAAG IWRRGIVGHP I KVVKGHVA ...String: MLAAEEGGAA IHVGHHTLVF ELFGMTFNGD TILATAVTAV IVIALAFYLR AKVTSTGVPS GVQLFWEALT IQMRQQIEGS IGMKIAPFV LPLSVTIFVF ILISNWLAVL PLQYGGADGA AAELYKAPAS DINFVLALAL FVFVCYHAAG IWRRGIVGHP I KVVKGHVA FLAPINIVEE LAKPISLALR LFGNIFAGGI LVALIAMFPW YIQWFPNAVW KTFDLFVGLI QAFIFSLLTI LY FSQSMEL DHEDH UniProtKB: ATP synthase subunit a |
-Macromolecule #2: ATP synthase subunit b
| Macromolecule | Name: ATP synthase subunit b / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Mycolicibacterium smegmatis (bacteria) Mycolicibacterium smegmatis (bacteria) |
| Molecular weight | Theoretical: 17.636701 KDa |
| Sequence | String: MGEFSATILA ASQAAEEGGG GSNFLIPNGT FFAVLIIFLI VLGVISKWVV PPISKVLAER EAMLAKTAAD NRKSAEQVAA AQADYEKEM AEARAQASAL RDEARAAGRS VVDEKRAQAS GEVAQTLTQA DQQLSAQGDQ VRSGLESSVD GLSAKLASRI L GVDVNSGG TQ UniProtKB: ATP synthase subunit b |
-Macromolecule #3: ATP synthase subunit b-delta
| Macromolecule | Name: ATP synthase subunit b-delta / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Mycolicibacterium smegmatis (bacteria) Mycolicibacterium smegmatis (bacteria) |
| Molecular weight | Theoretical: 47.504723 KDa |
| Sequence | String: MSIFIGQLIG FAVIAFIIVK WVVPPVRTLM RNQQEAVRAA LAESAEAAKK LADADAMHAK ALADAKAESE KVTEEAKQDS ERIAAQLSE QAGSEAERIK AQGAQQIQLM RQQLIRQLRT GLGAEAVNKA AEIVRAHVAD PQAQSATVDR FLSELEQMAP S SVVIDTAA ...String: MSIFIGQLIG FAVIAFIIVK WVVPPVRTLM RNQQEAVRAA LAESAEAAKK LADADAMHAK ALADAKAESE KVTEEAKQDS ERIAAQLSE QAGSEAERIK AQGAQQIQLM RQQLIRQLRT GLGAEAVNKA AEIVRAHVAD PQAQSATVDR FLSELEQMAP S SVVIDTAA TSRLRAASRQ SLAALVEKFD SVAGGLDADG LTNLADELAS VAKLLLSETA LNKHLAEPTD DSAPKVRLLE RL LSDKVSA TTLDLLRTAV SNRWSTESNL IDAVEHTARL ALLKRAEIAG EVDEVEEQLF RFGRVLDAEP RLSALLSDYT TPA EGRVAL LDKALTGRPG VNQTAAALLS QTVGLLRGER ADEAVIDLAE LAVSRRGEVV AHVSAAAELS DAQRTRLTEV LSRI YGRPV SVQLHVDPEL LGGLSITVGD EVIDGSIASR LAAAQTGLPD UniProtKB: ATP synthase subunit b-delta |
-Macromolecule #4: ATP synthase subunit c
| Macromolecule | Name: ATP synthase subunit c / type: protein_or_peptide / ID: 4 / Number of copies: 9 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Mycolicibacterium smegmatis (bacteria) Mycolicibacterium smegmatis (bacteria) |
| Molecular weight | Theoretical: 8.597982 KDa |
| Sequence | String: MDLDPNAIIT AGALIGGGLI MGGGAIGAGI GDGIAGNALI SGIARQPEAQ GRLFTPFFIT VGLVEAAYFI NLAFMALFVF ATPGLQ UniProtKB: ATP synthase subunit c |
-Macromolecule #5: Bedaquiline
| Macromolecule | Name: Bedaquiline / type: ligand / ID: 5 / Number of copies: 7 / Formula: BQ1 |
|---|---|
| Molecular weight | Theoretical: 555.505 Da |
| Chemical component information | 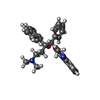 ChemComp-BQ1: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 6 mg/mL |
|---|---|
| Buffer | pH: 7.4 |
| Grid | Model: Homemade / Material: COPPER/RHODIUM / Support film - Material: CARBON / Support film - topology: HOLEY / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 120 sec. / Pretreatment - Atmosphere: OTHER |
| Vitrification | Cryogen name: ETHANE-PROPANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK III |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: FEI FALCON IV (4k x 4k) / Digitization - Dimensions - Width: 4096 pixel / Digitization - Dimensions - Height: 4096 pixel / Number real images: 7589 / Average electron dose: 41.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 50.0 µm / Calibrated defocus max: 3.0 µm / Calibrated defocus min: 0.6 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal magnification: 75000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Refinement | Space: RECIPROCAL / Protocol: AB INITIO MODEL |
|---|---|
| Output model |  PDB-7jgc: |
 Movie
Movie Controller
Controller






















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)