+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-20844 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Metavinculin ABD-F-actin complex | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | vinculin / metavinculin / actin / mechanobiology / mechanosensing / cytoskeleton / cell adhesion / focal adhesion | |||||||||
| Function / homology |  Function and homology information Function and homology informationregulation of protein localization to adherens junction / outer dense plaque of desmosome / inner dense plaque of desmosome / podosome ring / terminal web / cell-substrate junction / epithelial cell-cell adhesion / zonula adherens / fascia adherens / Striated Muscle Contraction ...regulation of protein localization to adherens junction / outer dense plaque of desmosome / inner dense plaque of desmosome / podosome ring / terminal web / cell-substrate junction / epithelial cell-cell adhesion / zonula adherens / fascia adherens / Striated Muscle Contraction / dystroglycan binding / alpha-catenin binding / cell-cell contact zone / apical junction assembly / costamere / regulation of establishment of endothelial barrier / axon extension / adherens junction assembly / protein localization to cell surface / lamellipodium assembly / regulation of focal adhesion assembly / maintenance of blood-brain barrier / brush border / striated muscle thin filament / skeletal muscle thin filament assembly / Smooth Muscle Contraction / skeletal muscle fiber development / stress fiber / negative regulation of cell migration / Turbulent (oscillatory, disturbed) flow shear stress activates signaling by PIEZO1 and integrins in endothelial cells / cell-matrix adhesion / morphogenesis of an epithelium / cell projection / adherens junction / actin filament / Signaling by high-kinase activity BRAF mutants / MAP2K and MAPK activation / beta-catenin binding / sarcolemma / Hydrolases; Acting on acid anhydrides; Acting on acid anhydrides to facilitate cellular and subcellular movement / platelet aggregation / specific granule lumen / Signaling by RAF1 mutants / Signaling by moderate kinase activity BRAF mutants / Paradoxical activation of RAF signaling by kinase inactive BRAF / Signaling downstream of RAS mutants / cell-cell junction / Signaling by BRAF and RAF1 fusions / Signaling by ALK fusions and activated point mutants / Platelet degranulation / actin cytoskeleton / extracellular vesicle / actin binding / secretory granule lumen / High laminar flow shear stress activates signaling by PIEZO1 and PECAM1:CDH5:KDR in endothelial cells / ficolin-1-rich granule lumen / molecular adaptor activity / cytoskeleton / cell adhesion / hydrolase activity / cadherin binding / membrane raft / focal adhesion / ubiquitin protein ligase binding / Neutrophil degranulation / structural molecule activity / protein-containing complex / extracellular exosome / extracellular region / ATP binding / plasma membrane / cytosol / cytoplasm Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) / Homo sapiens (human) /  | |||||||||
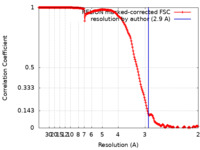
| Method | helical reconstruction / cryo EM / Resolution: 2.9 Å | |||||||||
 Authors Authors | Mei L / Alushin GM | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Elife / Year: 2020 Journal: Elife / Year: 2020Title: Molecular mechanism for direct actin force-sensing by α-catenin. Authors: Lin Mei / Santiago Espinosa de Los Reyes / Matthew J Reynolds / Rachel Leicher / Shixin Liu / Gregory M Alushin /  Abstract: The actin cytoskeleton mediates mechanical coupling between cells and their tissue microenvironments. The architecture and composition of actin networks are modulated by force; however, it is unclear ...The actin cytoskeleton mediates mechanical coupling between cells and their tissue microenvironments. The architecture and composition of actin networks are modulated by force; however, it is unclear how interactions between actin filaments (F-actin) and associated proteins are mechanically regulated. Here we employ both optical trapping and biochemical reconstitution with myosin motor proteins to show single piconewton forces applied solely to F-actin enhance binding by the human version of the essential cell-cell adhesion protein αE-catenin but not its homolog vinculin. Cryo-electron microscopy structures of both proteins bound to F-actin reveal unique rearrangements that facilitate their flexible C-termini refolding to engage distinct interfaces. Truncating α-catenin's C-terminus eliminates force-activated F-actin binding, and addition of this motif to vinculin confers force-activated binding, demonstrating that α-catenin's C-terminus is a modular detector of F-actin tension. Our studies establish that piconewton force on F-actin can enhance partner binding, which we propose mechanically regulates cellular adhesion through α-catenin. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_20844.map.gz emd_20844.map.gz | 1.3 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-20844-v30.xml emd-20844-v30.xml emd-20844.xml emd-20844.xml | 27.2 KB 27.2 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_20844_fsc.xml emd_20844_fsc.xml | 18 KB | Display |  FSC data file FSC data file |
| Images |  emd_20844.png emd_20844.png | 123.1 KB | ||
| Masks |  emd_20844_msk_1.map emd_20844_msk_1.map | 512 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-20844.cif.gz emd-20844.cif.gz | 8 KB | ||
| Others |  emd_20844_additional_1.map.gz emd_20844_additional_1.map.gz emd_20844_additional_2.map.gz emd_20844_additional_2.map.gz emd_20844_half_map_1.map.gz emd_20844_half_map_1.map.gz emd_20844_half_map_2.map.gz emd_20844_half_map_2.map.gz | 411.4 MB 285.9 MB 412.2 MB 412.4 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-20844 http://ftp.pdbj.org/pub/emdb/structures/EMD-20844 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-20844 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-20844 | HTTPS FTP |
-Validation report
| Summary document |  emd_20844_validation.pdf.gz emd_20844_validation.pdf.gz | 781.2 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_20844_full_validation.pdf.gz emd_20844_full_validation.pdf.gz | 780.8 KB | Display | |
| Data in XML |  emd_20844_validation.xml.gz emd_20844_validation.xml.gz | 23.4 KB | Display | |
| Data in CIF |  emd_20844_validation.cif.gz emd_20844_validation.cif.gz | 29.8 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-20844 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-20844 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-20844 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-20844 | HTTPS FTP |
-Related structure data
| Related structure data |  6upwMC  6upvC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | |
| EM raw data |  EMPIAR-10663 (Title: Metavinculin ABD-F-actin complex / Data size: 593.0 EMPIAR-10663 (Title: Metavinculin ABD-F-actin complex / Data size: 593.0 Data #1: unaligned multi-frame micrographs of metavinculin ABD-actin complex [micrographs - multiframe]) |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_20844.map.gz / Format: CCP4 / Size: 512 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_20844.map.gz / Format: CCP4 / Size: 512 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. generated in cubic-lattice coordinate | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.03 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Mask #1
| File |  emd_20844_msk_1.map emd_20844_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
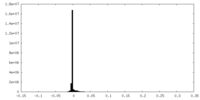
| Density Histograms |
-Additional map: unfiltered, unsharpened map
| File | emd_20844_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | unfiltered, unsharpened map | ||||||||||||
| Projections & Slices |
| ||||||||||||
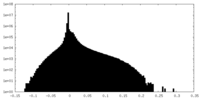
| Density Histograms |
-Additional map: B-factor sharpened, local resolution-filtered map
| File | emd_20844_additional_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | B-factor sharpened, local resolution-filtered map | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_20844_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
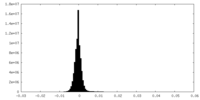
| Density Histograms |
-Half map: #2
| File | emd_20844_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
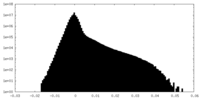
| Density Histograms |
- Sample components
Sample components
-Entire : metavinculin ABD-F-actin complex
| Entire | Name: metavinculin ABD-F-actin complex |
|---|---|
| Components |
|
-Supramolecule #1: metavinculin ABD-F-actin complex
| Supramolecule | Name: metavinculin ABD-F-actin complex / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#2 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 28.2 kDa/nm |
-Macromolecule #1: Vinculin
| Macromolecule | Name: Vinculin / type: protein_or_peptide / ID: 1 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 123.956375 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MPVFHTRTIE SILEPVAQQI SHLVIMHEEG EVDGKAIPDL TAPVAAVQAA VSNLVRVGKE TVQTTEDQIL KRDMPPAFIK VENACTKLV QAAQMLQSDP YSVPARDYLI DGSRGILSGT SDLLLTFDEA EVRKIIRVCK GILEYLTVAE VVETMEDLVT Y TKNLGPGM ...String: MPVFHTRTIE SILEPVAQQI SHLVIMHEEG EVDGKAIPDL TAPVAAVQAA VSNLVRVGKE TVQTTEDQIL KRDMPPAFIK VENACTKLV QAAQMLQSDP YSVPARDYLI DGSRGILSGT SDLLLTFDEA EVRKIIRVCK GILEYLTVAE VVETMEDLVT Y TKNLGPGM TKMAKMIDER QQELTHQEHR VMLVNSMNTV KELLPVLISA MKIFVTTKNS KNQGIEEALK NRNFTVEKMS AE INEIIRV LQLTSWDEDA WASKDTEAMK RALASIDSKL NQAKGWLRDP SASPGDAGEQ AIRQILDEAG KVGELCAGKE RRE ILGTCK MLGQMTDQVA DLRARGQGSS PVAMQKAQQV SQGLDVLTAK VENAARKLEA MTNSKQSIAK KIDAAQNWLA DPNG GPEGE EQIRGALAEA RKIAELCDDP KERDDILRSL GEISALTSKL ADLRRQGKGD SPEARALAKQ VATALQNLQT KTNRA VANS RPAKAAVHLE GKIEQAQRWI DNPTVDDRGV GQAAIRGLVA EGHRLANVMM GPYRQDLLAK CDRVDQLTAQ LADLAA RGE GESPQARALA SQLQDSLKDL KARMQEAMTQ EVSDVFSDTT TPIKLLAVAA TAPPDAPNRE EVFDERAANF ENHSGKL GA TAEKAAAVGT ANKSTVEGIQ ASVKTARELT PQVVSAARIL LRNPGNQAAY EHFETMKNQW IDNVEKMTGL VDEAIDTK S LLDASEEAIK KDLDKCKVAM ANIQPQMLVA GATSIARRAN RILLVAKREV ENSEDPKFRE AVKAASDELS KTISPMVMD AKAVAGNISD PGLQKSFLDS GYRILGAVAK VREAFQPQEP DFPPPPPDLE QLRLTDELAP PKPPLPEGEV PPPRPPPPEE KDEEFPEQK AGEVINQPMM MAARQLHDEA RKWSSKPGIP AAEVGIGVVA EADAADAAGF PVPPDMEDDY EPELLLMPSN Q PVNQPILA AAQSLHREAT KWSSKGNDII AAAKRMALLM AEMSRLVRGG SGTKRALIQC AKDIAKASDE VTRLAKEVAK QC TDKRIRT NLLQVCERIP TISTQLKILS TVKATMLGRT NISDEESEQA TEMLVHNAQN LMQSVKETVR EAEAASIKIR TDA GFTLRW VRKTPWYQ UniProtKB: Vinculin |
-Macromolecule #2: Actin, alpha skeletal muscle
| Macromolecule | Name: Actin, alpha skeletal muscle / type: protein_or_peptide / ID: 2 / Number of copies: 5 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 41.875633 KDa |
| Sequence | String: DEDETTALVC DNGSGLVKAG FAGDDAPRAV FPSIVGRPRH QGVMVGMGQK DSYVGDEAQS KRGILTLKYP IE(HIC)GII TNW DDMEKIWHHT FYNELRVAPE EHPTLLTEAP LNPKANREKM TQIMFETFNV PAMYVAIQAV LSLYASGRTT GIVLDSG DG VTHNVPIYEG ...String: DEDETTALVC DNGSGLVKAG FAGDDAPRAV FPSIVGRPRH QGVMVGMGQK DSYVGDEAQS KRGILTLKYP IE(HIC)GII TNW DDMEKIWHHT FYNELRVAPE EHPTLLTEAP LNPKANREKM TQIMFETFNV PAMYVAIQAV LSLYASGRTT GIVLDSG DG VTHNVPIYEG YALPHAIMRL DLAGRDLTDY LMKILTERGY SFVTTAEREI VRDIKEKLCY VALDFENEMA TAASSSSL E KSYELPDGQV ITIGNERFRC PETLFQPSFI GMESAGIHET TYNSIMKCDI DIRKDLYANN VMSGGTTMYP GIADRMQKE ITALAPSTMK IKIIAPPERK YSVWIGGSIL ASLSTFQQMW ITKQEYDEAG PSIVHRKCF UniProtKB: Actin, alpha skeletal muscle |
-Macromolecule #3: ADENOSINE-5'-DIPHOSPHATE
| Macromolecule | Name: ADENOSINE-5'-DIPHOSPHATE / type: ligand / ID: 3 / Number of copies: 5 / Formula: ADP |
|---|---|
| Molecular weight | Theoretical: 427.201 Da |
| Chemical component information |  ChemComp-ADP: |
-Macromolecule #4: MAGNESIUM ION
| Macromolecule | Name: MAGNESIUM ION / type: ligand / ID: 4 / Number of copies: 5 / Formula: MG |
|---|---|
| Molecular weight | Theoretical: 24.305 Da |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | helical reconstruction |
| Aggregation state | helical array |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: SUPER-RESOLUTION / Digitization - Frames/image: 1-40 / Number grids imaged: 1 / Average exposure time: 10.0 sec. / Average electron dose: 60.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller






















 X (Sec.)
X (Sec.) Y (Row.)
Y (Row.) Z (Col.)
Z (Col.)