+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-20575 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of RET/GFRa1/GDNF extracellular complex | |||||||||
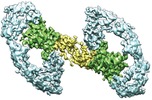
 Map data Map data | Cryo-EM structure of RET/GFR%u03B11/GDNF extracellular complex. The 3D refinement was applied with C2 symmetry. | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | RET / receptor tyrosine kinase / cryo-EM / SIGNALING PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationchemoattractant activity involved in axon guidance / mesenchymal to epithelial transition involved in metanephros morphogenesis / dorsal spinal cord development / positive regulation of mesenchymal to epithelial transition involved in metanephros morphogenesis / ureteric bud formation / positive regulation of ureteric bud formation / regulation of semaphorin-plexin signaling pathway / postganglionic parasympathetic fiber development / glial cell-derived neurotrophic factor receptor activity / glial cell-derived neurotrophic factor receptor binding ...chemoattractant activity involved in axon guidance / mesenchymal to epithelial transition involved in metanephros morphogenesis / dorsal spinal cord development / positive regulation of mesenchymal to epithelial transition involved in metanephros morphogenesis / ureteric bud formation / positive regulation of ureteric bud formation / regulation of semaphorin-plexin signaling pathway / postganglionic parasympathetic fiber development / glial cell-derived neurotrophic factor receptor activity / glial cell-derived neurotrophic factor receptor binding / postsynaptic membrane organization / Peyer's patch morphogenesis / GDF15-GFRAL signaling pathway / positive regulation of metanephric glomerulus development / ureter maturation / embryonic epithelial tube formation / glial cell-derived neurotrophic factor receptor signaling pathway / lymphocyte migration into lymphoid organs / posterior midgut development / regulation of morphogenesis of a branching structure / neurotrophin receptor activity / regulation of dopamine uptake involved in synaptic transmission / Formation of the ureteric bud / membrane protein proteolysis / Formation of the nephric duct / peristalsis / neuron cell-cell adhesion / enteric nervous system development / positive regulation of dopamine secretion / sympathetic nervous system development / positive regulation of branching involved in ureteric bud morphogenesis / commissural neuron axon guidance / organ induction / peripheral nervous system development / metanephros development / NCAM1 interactions / neuron maturation / plasma membrane protein complex / regulation of stem cell differentiation / positive regulation of extrinsic apoptotic signaling pathway in absence of ligand / mRNA stabilization / positive regulation of cell adhesion mediated by integrin / neural crest cell migration / extrinsic component of membrane / ureteric bud development / branching involved in ureteric bud morphogenesis / response to pain / regulation of axonogenesis / homophilic cell-cell adhesion / RET signaling / positive regulation of cell size / embryonic organ development / negative regulation of extrinsic apoptotic signaling pathway in absence of ligand / regulation of cell adhesion / cellular response to retinoic acid / NPAS4 regulates expression of target genes / multivesicular body / transmembrane receptor protein tyrosine kinase activity / axon guidance / cell surface receptor protein tyrosine kinase signaling pathway / adult locomotory behavior / growth factor activity / positive regulation of cell differentiation / kidney development / positive regulation of neuron projection development / receptor protein-tyrosine kinase / receptor tyrosine kinase binding / integrin binding / male gonad development / neuron projection development / cell migration / nervous system development / signaling receptor activity / MAPK cascade / regulation of gene expression / RAF/MAP kinase cascade / protein tyrosine kinase activity / negative regulation of neuron apoptotic process / cell surface receptor signaling pathway / positive regulation of phosphatidylinositol 3-kinase/protein kinase B signal transduction / receptor complex / positive regulation of MAPK cascade / endosome membrane / positive regulation of cell migration / signaling receptor binding / axon / external side of plasma membrane / neuronal cell body / positive regulation of cell population proliferation / calcium ion binding / positive regulation of gene expression / negative regulation of apoptotic process / positive regulation of DNA-templated transcription / Golgi apparatus / signal transduction / protein homodimerization activity / positive regulation of transcription by RNA polymerase II / extracellular space / extracellular exosome / extracellular region Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 4.4 Å | |||||||||
 Authors Authors | Li J / Shang GJ | |||||||||
 Citation Citation |  Journal: Elife / Year: 2019 Journal: Elife / Year: 2019Title: Cryo-EM analyses reveal the common mechanism and diversification in the activation of RET by different ligands. Authors: Jie Li / Guijun Shang / Yu-Ju Chen / Chad A Brautigam / Jen Liou / Xuewu Zhang / Xiao-Chen Bai /  Abstract: RET is a receptor tyrosine kinase (RTK) that plays essential roles in development and has been implicated in several human diseases. Different from most of RTKs, RET requires not only its cognate ...RET is a receptor tyrosine kinase (RTK) that plays essential roles in development and has been implicated in several human diseases. Different from most of RTKs, RET requires not only its cognate ligands but also co-receptors for activation, the mechanisms of which remain unclear due to lack of high-resolution structures of the ligand/co-receptor/receptor complexes. Here, we report cryo-EM structures of the extracellular region ternary complexes of GDF15/GFRAL/RET, GDNF/GFRα1/RET, NRTN/GFRα2/RET and ARTN/GFRα3/RET. These structures reveal that all the four ligand/co-receptor pairs, while using different atomic interactions, induce a specific dimerization mode of RET that is poised to bring the two kinase domains into close proximity for cross-phosphorylation. The NRTN/GFRα2/RET dimeric complex further pack into a tetrameric assembly, which is shown by our cell-based assays to regulate the endocytosis of RET. Our analyses therefore reveal both the common mechanism and diversification in the activation of RET by different ligands. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_20575.map.gz emd_20575.map.gz | 38.1 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-20575-v30.xml emd-20575-v30.xml emd-20575.xml emd-20575.xml | 15.1 KB 15.1 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_20575.png emd_20575.png | 319.2 KB | ||
| Filedesc metadata |  emd-20575.cif.gz emd-20575.cif.gz | 6.2 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-20575 http://ftp.pdbj.org/pub/emdb/structures/EMD-20575 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-20575 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-20575 | HTTPS FTP |
-Validation report
| Summary document |  emd_20575_validation.pdf.gz emd_20575_validation.pdf.gz | 579.3 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_20575_full_validation.pdf.gz emd_20575_full_validation.pdf.gz | 578.8 KB | Display | |
| Data in XML |  emd_20575_validation.xml.gz emd_20575_validation.xml.gz | 5.7 KB | Display | |
| Data in CIF |  emd_20575_validation.cif.gz emd_20575_validation.cif.gz | 6.6 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-20575 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-20575 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-20575 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-20575 | HTTPS FTP |
-Related structure data
| Related structure data |  6q2nMC  6q2jC  6q2oC  6q2rC  6q2sC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_20575.map.gz / Format: CCP4 / Size: 40.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_20575.map.gz / Format: CCP4 / Size: 40.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Cryo-EM structure of RET/GFR%u03B11/GDNF extracellular complex. The 3D refinement was applied with C2 symmetry. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.07 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : RET, GFRAL and GDF15 extracellular complex
| Entire | Name: RET, GFRAL and GDF15 extracellular complex |
|---|---|
| Components |
|
-Supramolecule #1: RET, GFRAL and GDF15 extracellular complex
| Supramolecule | Name: RET, GFRAL and GDF15 extracellular complex / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#3 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 200 kDa/nm |
-Macromolecule #1: Glial cell line-derived neurotrophic factor
| Macromolecule | Name: Glial cell line-derived neurotrophic factor / type: protein_or_peptide / ID: 1 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 15.096242 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: SPDKQMAVLP RRERNRQAAA ANPENSRGKG RRGQRGKNRG CVLTAIHLNV TDLGLGYETK EELIFRYCSG SCDAAETTYD KILKNLSRN RRLVSDKVGQ ACCRPIAFDD DLSFLDDNLV YHILRKHSAK RCGCI UniProtKB: Glial cell line-derived neurotrophic factor |
-Macromolecule #2: GDNF family receptor alpha-1
| Macromolecule | Name: GDNF family receptor alpha-1 / type: protein_or_peptide / ID: 2 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 46.358551 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: DRLDCVKASD QCLKEQSCST KYRTLRQCVA GKETNFSLAS GLEAKDECRS AMEALKQKSL YNCRCKRGMK KEKNCLRIYW SMYQSLQGN DLLEDSPYEP VNSRLSDIFR VVPFISDVFQ QVEHIPKGNN CLDAAKACNL DDICKKYRSA YITPCTTSVS N DVCNRRKC ...String: DRLDCVKASD QCLKEQSCST KYRTLRQCVA GKETNFSLAS GLEAKDECRS AMEALKQKSL YNCRCKRGMK KEKNCLRIYW SMYQSLQGN DLLEDSPYEP VNSRLSDIFR VVPFISDVFQ QVEHIPKGNN CLDAAKACNL DDICKKYRSA YITPCTTSVS N DVCNRRKC HKALRQFFDK VPAKHSYGML FCSCRDIACT ERRRQTIVPV CSYEEREKPN CLNLQDSCKT NYICRSRLAD FF TNCQPES RSVSSCLKEN YADCLLAYSG LIGTVMTPNY IDSSSLSVAP WCDCSNSGND LEECLKFLNF FKDNTCLKNA IQA FGNGSD VTVWQPAFPV QTTTATTTTA LRVKNKPLGP AGSENEIPTH VLPPCANLQA QKLKSNVSGN THLCISNGNY EKEG LGGTH HHHHHHH UniProtKB: GDNF family receptor alpha-1 |
-Macromolecule #3: Proto-oncogene tyrosine-protein kinase receptor Ret
| Macromolecule | Name: Proto-oncogene tyrosine-protein kinase receptor Ret / type: protein_or_peptide / ID: 3 / Number of copies: 2 / Enantiomer: LEVO / EC number: receptor protein-tyrosine kinase |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 69.100812 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: LYFSRDAYWE KLYVDQAAGT PLLYVHALRD APEEVPSFRL GQHLYGTYRT RLHENNWICI QEDTGLLYLN RSLDHSSWEK LSVRNHGFP LLTVYLKVFL SPTSLREGEC QWPGCARVYF SFFNTSFPAC SSLKPRELCF PETRPSFRIR ENRPPGTFHQ F RLLPVQFL ...String: LYFSRDAYWE KLYVDQAAGT PLLYVHALRD APEEVPSFRL GQHLYGTYRT RLHENNWICI QEDTGLLYLN RSLDHSSWEK LSVRNHGFP LLTVYLKVFL SPTSLREGEC QWPGCARVYF SFFNTSFPAC SSLKPRELCF PETRPSFRIR ENRPPGTFHQ F RLLPVQFL CPNISVAYRL LEGEGLPFRC APDSLEVSTR WALDREQREK YELVAVCTVH AGAREEVVMV PFPVTVYDED DS APTFPAG VDTASAVVEF KRKEDTVVAT LRVFDADVVP ASGELVRRYT STLLPGDTWA QQTFRVEHWP NETSVQANGS FVR ATVHDY RLVLNRNLSI SENRTMQLAV LVNDSDFQGP GAGVLLLHFN VSVLPVSLHL PSTYSLSVSR RARRFAQIGK VCVE NCQAF SGINVQYKLH SSGANCSTLG VVTSAEDTSG ILFVNDTKAL RRPKCAELHY MVVATDQQTS RQAQAQLLVT VEGSY VAEE AGCPLSCAVS KRRLECEECG GLGSPTGRCE WRQGDGKGIT RNFSTCSPST KTCPDGHCDV VETQDINICP QDCLRG SIV GGHEPGEPRG IKAGYGTCNC FPEEEKCFCE PEDIQDPLCD ELCRGTHHHH HHHH UniProtKB: Proto-oncogene tyrosine-protein kinase receptor Ret |
-Macromolecule #4: CALCIUM ION
| Macromolecule | Name: CALCIUM ION / type: ligand / ID: 4 / Number of copies: 8 / Formula: CA |
|---|---|
| Molecular weight | Theoretical: 40.078 Da |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 1 mg/mL |
|---|---|
| Buffer | pH: 7.4 |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Specialist optics | Energy filter - Name: GIF Quantum LS / Energy filter - Slit width: 20 eV |
| Image recording | Film or detector model: GATAN K2 QUANTUM (4k x 4k) / Detector mode: SUPER-RESOLUTION / Digitization - Frames/image: 1-30 / Number grids imaged: 1 / Average exposure time: 15.0 sec. / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 70.0 µm / Calibrated magnification: 46729 / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Refinement | Space: REAL / Protocol: FLEXIBLE FIT / Overall B value: 190 |
|---|---|
| Output model |  PDB-6q2n: |
 Movie
Movie Controller
Controller
























 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)





















