[English] 日本語
 Yorodumi
Yorodumi- EMDB-20458: Rhinovirus C15 complexed with domains I and II of receptor CDHR3 -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-20458 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Rhinovirus C15 complexed with domains I and II of receptor CDHR3 | |||||||||
 Map data Map data | Rhinovirus C15 complexed with domains I and II of receptor CDHR3 | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | receptor / cadherin / VIRUS-CELL ADHESION complex | |||||||||
| Function / homology |  Function and homology information Function and homology informationcalcium-dependent cell-cell adhesion / cell-cell adhesion mediated by cadherin / adherens junction organization / catenin complex / cell-cell junction assembly / homophilic cell-cell adhesion / symbiont-mediated suppression of host cytoplasmic pattern recognition receptor signaling pathway via inhibition of RIG-I activity / picornain 2A / symbiont-mediated suppression of host mRNA export from nucleus / adherens junction ...calcium-dependent cell-cell adhesion / cell-cell adhesion mediated by cadherin / adherens junction organization / catenin complex / cell-cell junction assembly / homophilic cell-cell adhesion / symbiont-mediated suppression of host cytoplasmic pattern recognition receptor signaling pathway via inhibition of RIG-I activity / picornain 2A / symbiont-mediated suppression of host mRNA export from nucleus / adherens junction / symbiont genome entry into host cell via pore formation in plasma membrane / picornain 3C / T=pseudo3 icosahedral viral capsid / beta-catenin binding / host cell cytoplasmic vesicle membrane / cell morphogenesis / ribonucleoside triphosphate phosphatase activity / cell migration / nucleoside-triphosphate phosphatase / channel activity / virus receptor activity / monoatomic ion transmembrane transport / DNA replication / RNA helicase activity / cadherin binding / endocytosis involved in viral entry into host cell / symbiont-mediated activation of host autophagy / RNA-directed RNA polymerase / cysteine-type endopeptidase activity / viral RNA genome replication / RNA-directed RNA polymerase activity / calcium ion binding / DNA-templated transcription / virion attachment to host cell / host cell nucleus / structural molecule activity / proteolysis / RNA binding / zinc ion binding / ATP binding / membrane / plasma membrane Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) / Homo sapiens (human) /  Rhinovirus C Rhinovirus C | |||||||||
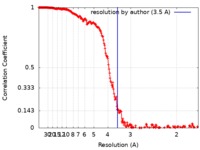
| Method | single particle reconstruction / cryo EM / Resolution: 3.5 Å | |||||||||
 Authors Authors | Sun Y / Watters K | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Proc Natl Acad Sci U S A / Year: 2020 Journal: Proc Natl Acad Sci U S A / Year: 2020Title: Cryo-EM structure of rhinovirus C15a bound to its cadherin-related protein 3 receptor. Authors: Yingyuan Sun / Kelly Watters / Marchel G Hill / Qianglin Fang / Yue Liu / Richard J Kuhn / Thomas Klose / Michael G Rossmann / Ann C Palmenberg /  Abstract: Infection by (RV-C), a species of Picornaviridae , is strongly associated with childhood asthma exacerbations. Cellular binding and entry by all RV-C, which trigger these episodes, is mediated by ...Infection by (RV-C), a species of Picornaviridae , is strongly associated with childhood asthma exacerbations. Cellular binding and entry by all RV-C, which trigger these episodes, is mediated by the first extracellular domain (EC1) of cadherin-related protein 3 (CDHR3), a surface cadherin-like protein expressed primarily on the apical surfaces of ciliated airway epithelial cells. Although recombinant EC1 is a potent inhibitor of viral infection, there is no molecular description of this protein or its binding site on RV-C. Here we present cryo-electron microscopy (EM) data resolving the EC1 and EC1+2 domains of human CDHR3 complexed with viral isolate C15a. Structure-suggested residues contributing to required interfaces on both EC1 and C15a were probed and identified by mutagenesis studies with four different RV-C genotypes. In contrast to most other rhinoviruses, which bind intercellular adhesion molecule 1 receptors via a capsid protein VP1-specific fivefold canyon feature, the CDHR3 EC1 contacts C15a, and presumably all RV-Cs, in a unique cohesive footprint near the threefold vertex, encompassing residues primarily from viral protein VP3, but also from VP1 and VP2. The EC1+2 footprint on C15a is similar to that of EC1 alone but shows that steric hindrance imposed by EC2 would likely prevent multiprotein binding by the native receptor at any singular threefold vertex. Definition of the molecular interface between the RV-Cs and their receptors provides new avenues that can be explored for potential antiviral therapies. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_20458.map.gz emd_20458.map.gz | 635.4 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-20458-v30.xml emd-20458-v30.xml emd-20458.xml emd-20458.xml | 24 KB 24 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_20458_fsc.xml emd_20458_fsc.xml | 23.8 KB | Display |  FSC data file FSC data file |
| Images |  emd_20458.png emd_20458.png | 259 KB | ||
| Masks |  emd_20458_msk_1.map emd_20458_msk_1.map | 729 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-20458.cif.gz emd-20458.cif.gz | 7.1 KB | ||
| Others |  emd_20458_half_map_1.map.gz emd_20458_half_map_1.map.gz emd_20458_half_map_2.map.gz emd_20458_half_map_2.map.gz | 183.6 MB 183.6 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-20458 http://ftp.pdbj.org/pub/emdb/structures/EMD-20458 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-20458 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-20458 | HTTPS FTP |
-Related structure data
| Related structure data |  6psfMC  6ppoC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_20458.map.gz / Format: CCP4 / Size: 729 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_20458.map.gz / Format: CCP4 / Size: 729 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Rhinovirus C15 complexed with domains I and II of receptor CDHR3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.865 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Mask #1
| File |  emd_20458_msk_1.map emd_20458_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Rhinovirus C15 complexed with domains I and II...
| File | emd_20458_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Rhinovirus C15 complexed with domains I and II of receptor CDHR3, half map 1 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Rhinovirus C15 complexed with domains I and II...
| File | emd_20458_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Rhinovirus C15 complexed with domains I and II of receptor CDHR3, half map 2 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Complex of rhinovirus C15 with domain I and domain II (EC1-2) fro...
| Entire | Name: Complex of rhinovirus C15 with domain I and domain II (EC1-2) from human CDHR3 |
|---|---|
| Components |
|
-Supramolecule #1: Complex of rhinovirus C15 with domain I and domain II (EC1-2) fro...
| Supramolecule | Name: Complex of rhinovirus C15 with domain I and domain II (EC1-2) from human CDHR3 type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Molecular weight | Theoretical: 6 MDa |
-Supramolecule #3: domain I and domain II (EC1-2) from human CDHR3
| Supramolecule | Name: domain I and domain II (EC1-2) from human CDHR3 / type: complex / ID: 3 / Parent: 1 / Macromolecule list: #5 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Supramolecule #2: Rhinovirus C
| Supramolecule | Name: Rhinovirus C / type: virus / ID: 2 / Parent: 1 / Macromolecule list: #1-#4 / NCBI-ID: 463676 / Sci species name: Rhinovirus C / Virus type: VIRION / Virus isolate: STRAIN / Virus enveloped: No / Virus empty: No |
|---|---|
| Host (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Capsid protein VP1
| Macromolecule | Name: Capsid protein VP1 / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO / EC number: picornain 2A |
|---|---|
| Source (natural) | Organism:  Rhinovirus C Rhinovirus C |
| Molecular weight | Theoretical: 31.802623 KDa |
| Sequence | String: NNDDPVENFV ESTLKEVLVV PDTKPSGPQH TTKPSILGAM EIGASSNATP ESTIETRYVY NTNTNAEADV EMFLGRSALW GKVTLTRQY AKWEINFQEQ AHIRKKFEFF TYLRFDMEVT IVTNNKGLMQ IMFVPPGIDH PETHDDRKWD SASNPSVFFQ P KSGFPRFT ...String: NNDDPVENFV ESTLKEVLVV PDTKPSGPQH TTKPSILGAM EIGASSNATP ESTIETRYVY NTNTNAEADV EMFLGRSALW GKVTLTRQY AKWEINFQEQ AHIRKKFEFF TYLRFDMEVT IVTNNKGLMQ IMFVPPGIDH PETHDDRKWD SASNPSVFFQ P KSGFPRFT IPFTGLASAY YMFYDGYDKP KGSDNNEYGI APTNDMGLLC FRTLDNSGGN DVKIYVKPKH ITAWVPRPPR AT QYTHKYS TNYHYKPNSS GPDEHVLKDR HFIKTRPLIS SA UniProtKB: Genome polyprotein |
-Macromolecule #2: Capsid protein VP3
| Macromolecule | Name: Capsid protein VP3 / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO / EC number: picornain 2A |
|---|---|
| Source (natural) | Organism:  Rhinovirus C Rhinovirus C |
| Molecular weight | Theoretical: 25.965037 KDa |
| Sequence | String: GLPTRLPSGS QQFMTTEDEQ SPNILPGFHP SKKIHIPGMI TNVMHMARVD SFIPINNIQG EVGKVSMYYI TVTKKTVTER ILVLPLEMS NTLFATTLLG EVLNYYANWS GSITITFMCV CDAFSTGKFL VAYTPPGGKL PEDRKQAMLG VHIIWDLGLQ S SCTIVVPW ...String: GLPTRLPSGS QQFMTTEDEQ SPNILPGFHP SKKIHIPGMI TNVMHMARVD SFIPINNIQG EVGKVSMYYI TVTKKTVTER ILVLPLEMS NTLFATTLLG EVLNYYANWS GSITITFMCV CDAFSTGKFL VAYTPPGGKL PEDRKQAMLG VHIIWDLGLQ S SCTIVVPW ISSGFYRRTK ADSFTHGGYV SLWYQTAFVP PVSGGTGSIL ATCSACPDMS VRMLRDSPMM EQKNELQ UniProtKB: Genome polyprotein |
-Macromolecule #3: Capsid protein VP2
| Macromolecule | Name: Capsid protein VP2 / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO / EC number: picornain 2A |
|---|---|
| Source (natural) | Organism:  Rhinovirus C Rhinovirus C |
| Molecular weight | Theoretical: 29.090658 KDa |
| Sequence | String: SPSVEACGYS DRLKQITIGN STITTQDSLH TVLAYGEWPT YLSDIDATSV DKPTHPETSA DRFYTLDSVE WQVGSHGWWW KLPDALKDM GVFGQNMYYH SMGRSGFIIH TQCNATKFHS GALIVAVIPE HQLAYVGGVK VNVGYDHTHP GQSGHQIRGP S QSNDRSGG ...String: SPSVEACGYS DRLKQITIGN STITTQDSLH TVLAYGEWPT YLSDIDATSV DKPTHPETSA DRFYTLDSVE WQVGSHGWWW KLPDALKDM GVFGQNMYYH SMGRSGFIIH TQCNATKFHS GALIVAVIPE HQLAYVGGVK VNVGYDHTHP GQSGHQIRGP S QSNDRSGG KPDEDPLFNC NGTLLGNITI FPHQIINLRT NNSSTIVVPY INCVPMDNML KHNNLSLVII PLVPLRPGSS GI NSVPITV TIAPYKSEFS GAMEAQRQ UniProtKB: Genome polyprotein |
-Macromolecule #4: Capsid protein VP4
| Macromolecule | Name: Capsid protein VP4 / type: protein_or_peptide / ID: 4 / Number of copies: 1 / Enantiomer: LEVO / EC number: picornain 2A |
|---|---|
| Source (natural) | Organism:  Rhinovirus C Rhinovirus C |
| Molecular weight | Theoretical: 7.174758 KDa |
| Sequence | String: GAQVSRQNNG THENGVTASN GSVIKYFNIN YYKDSASSGL SRQDFSQDPS KFTQPLVDTL TNPALM UniProtKB: Genome polyprotein |
-Macromolecule #5: Cadherin-related family member 3
| Macromolecule | Name: Cadherin-related family member 3 / type: protein_or_peptide / ID: 5 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 26.54357 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MASDYKDDDD KLHLILLPAT GNVAENSPPG TSVHKFSVKL SASLSPVIPG FPQIVNSNPL TEAFRVNWLS GTYFEVVTTG MEQLDFETG PNIFDLQIYV KDEVGVTDLQ VLTVQVTDVN EPPQFQGNLA EGLHLYIVER ANPGFIYQVE AFDPEDTSRN I PLSYFLIS ...String: MASDYKDDDD KLHLILLPAT GNVAENSPPG TSVHKFSVKL SASLSPVIPG FPQIVNSNPL TEAFRVNWLS GTYFEVVTTG MEQLDFETG PNIFDLQIYV KDEVGVTDLQ VLTVQVTDVN EPPQFQGNLA EGLHLYIVER ANPGFIYQVE AFDPEDTSRN I PLSYFLIS PPKSFRMSAN GTLFSTTELD FEAGHRSFHL IVEVRDSGGL KASTELQVNI VNLNDEVPRF TGGTKHHHHH H UniProtKB: Cadherin-related family member 3 |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.5 mg/mL |
|---|---|
| Buffer | pH: 7.4 |
| Grid | Support film - Material: CARBON / Support film - topology: LACEY / Details: unspecified |
| Vitrification | Cryogen name: NITROGEN / Chamber humidity: 80 % / Chamber temperature: 298 K / Instrument: GATAN CRYOPLUNGE 3 Details: 3 second blotting time. Instrument placed in BSL2 hood.. |
| Details | Recombinantly expressed EC1-2 was incubated with RV-C viruses overnight at 4 degrees C. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Specialist optics | Energy filter - Name: GIF Quantum LS / Energy filter - Slit width: 20 eV |
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: SUPER-RESOLUTION / Digitization - Dimensions - Width: 3838 pixel / Digitization - Dimensions - Height: 3710 pixel / Digitization - Frames/image: 1-60 / Number grids imaged: 1 / Number real images: 2452 / Average exposure time: 12.0 sec. / Average electron dose: 32.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 100.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 3.0 µm / Nominal defocus min: 1.0 µm / Nominal magnification: 81000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller

















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)