[English] 日本語
 Yorodumi
Yorodumi- EMDB-9827: The cryo-EM structure of HAV bound to a neutralizing antibody-F4 -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-9827 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
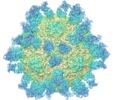
| Title | The cryo-EM structure of HAV bound to a neutralizing antibody-F4 | ||||||||||||
 Map data Map data | map of F4_Fab_HAV complex | ||||||||||||
 Sample Sample |
| ||||||||||||
 Keywords Keywords | Icosahedral symmetry / neutralizing antibody / HAV / complex / VIRUS | ||||||||||||
| Function / homology |  Function and homology information Function and homology informationhost cell mitochondrial outer membrane / symbiont-mediated suppression of host cytoplasmic pattern recognition receptor signaling pathway via inhibition of MAVS activity / picornain 3C / T=pseudo3 icosahedral viral capsid / ribonucleoside triphosphate phosphatase activity / host cell cytoplasmic vesicle membrane / host multivesicular body / nucleoside-triphosphate phosphatase / channel activity / monoatomic ion transmembrane transport ...host cell mitochondrial outer membrane / symbiont-mediated suppression of host cytoplasmic pattern recognition receptor signaling pathway via inhibition of MAVS activity / picornain 3C / T=pseudo3 icosahedral viral capsid / ribonucleoside triphosphate phosphatase activity / host cell cytoplasmic vesicle membrane / host multivesicular body / nucleoside-triphosphate phosphatase / channel activity / monoatomic ion transmembrane transport / RNA helicase activity / RNA-directed RNA polymerase / cysteine-type endopeptidase activity / viral RNA genome replication / RNA-directed RNA polymerase activity / DNA-templated transcription / symbiont entry into host cell / virion attachment to host cell / structural molecule activity / proteolysis / RNA binding / ATP binding / membrane Similarity search - Function | ||||||||||||
| Biological species |   Human hepatitis A virus Hu/Australia/HM175/1976 Human hepatitis A virus Hu/Australia/HM175/1976 | ||||||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.9 Å | ||||||||||||
 Authors Authors | Cao L / Liu P | ||||||||||||
| Funding support |  China, 3 items China, 3 items
| ||||||||||||
 Citation Citation |  Journal: PLoS Biol / Year: 2019 Journal: PLoS Biol / Year: 2019Title: Structural basis for neutralization of hepatitis A virus informs a rational design of highly potent inhibitors. Authors: Lei Cao / Pi Liu / Pan Yang / Qiang Gao / Hong Li / Yao Sun / Ling Zhu / Jianping Lin / Dan Su / Zihe Rao / Xiangxi Wang /  Abstract: Hepatitis A virus (HAV), an enigmatic and ancient pathogen, is a major causative agent of acute viral hepatitis worldwide. Although there are effective vaccines, antivirals against HAV infection are ...Hepatitis A virus (HAV), an enigmatic and ancient pathogen, is a major causative agent of acute viral hepatitis worldwide. Although there are effective vaccines, antivirals against HAV infection are still required, especially during fulminant hepatitis outbreaks. A more in-depth understanding of the antigenic characteristics of HAV and the mechanisms of neutralization could aid in the development of rationally designed antiviral drugs targeting HAV. In this paper, 4 new antibodies-F4, F6, F7, and F9-are reported that potently neutralize HAV at 50% neutralizing concentration values (neut50) ranging from 0.1 nM to 0.85 nM. High-resolution cryo-electron microscopy (cryo-EM) structures of HAV bound to F4, F6, F7, and F9, together with results of our previous studies on R10 fragment of antigen binding (Fab)-HAV complex, shed light on the locations and nature of the epitopes recognized by the 5 neutralizing monoclonal antibodies (NAbs). All the epitopes locate within the same patch and are highly conserved. The key structure-activity correlates based on the antigenic sites have been established. Based on the structural data of the single conserved antigenic site and key structure-activity correlates, one promising drug candidate named golvatinib was identified by in silico docking studies. Cell-based antiviral assays confirmed that golvatinib is capable of blocking HAV infection effectively with a 50% inhibitory concentration (IC50) of approximately 1 μM. These results suggest that the single conserved antigenic site from complete HAV capsid is a good antiviral target and that golvatinib could function as a lead compound for anti-HAV drug development. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_9827.map.gz emd_9827.map.gz | 181.5 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-9827-v30.xml emd-9827-v30.xml emd-9827.xml emd-9827.xml | 17.1 KB 17.1 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_9827.png emd_9827.png | 333.7 KB | ||
| Filedesc metadata |  emd-9827.cif.gz emd-9827.cif.gz | 6.6 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-9827 http://ftp.pdbj.org/pub/emdb/structures/EMD-9827 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-9827 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-9827 | HTTPS FTP |
-Validation report
| Summary document |  emd_9827_validation.pdf.gz emd_9827_validation.pdf.gz | 726.8 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_9827_full_validation.pdf.gz emd_9827_full_validation.pdf.gz | 726.3 KB | Display | |
| Data in XML |  emd_9827_validation.xml.gz emd_9827_validation.xml.gz | 6.8 KB | Display | |
| Data in CIF |  emd_9827_validation.cif.gz emd_9827_validation.cif.gz | 8 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-9827 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-9827 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-9827 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-9827 | HTTPS FTP |
-Related structure data
| Related structure data |  6jhqMC  9828C  9829C  9830C  6jhrC  6jhsC  6jhtC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_9827.map.gz / Format: CCP4 / Size: 193.2 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_9827.map.gz / Format: CCP4 / Size: 193.2 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | map of F4_Fab_HAV complex | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.35 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Human hepatitis A virus Hu/Australia/HM175/1976 and antibody-F4
| Entire | Name: Human hepatitis A virus Hu/Australia/HM175/1976 and antibody-F4 |
|---|---|
| Components |
|
-Supramolecule #1: Human hepatitis A virus Hu/Australia/HM175/1976 and antibody-F4
| Supramolecule | Name: Human hepatitis A virus Hu/Australia/HM175/1976 and antibody-F4 type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|
-Supramolecule #3: antibody-F4
| Supramolecule | Name: antibody-F4 / type: complex / ID: 3 / Parent: 1 / Macromolecule list: #4-#5 |
|---|---|
| Source (natural) | Organism:  |
-Supramolecule #2: Human hepatitis A virus Hu/Australia/HM175/1976
| Supramolecule | Name: Human hepatitis A virus Hu/Australia/HM175/1976 / type: virus / ID: 2 / Parent: 1 / Macromolecule list: #1-#3 / NCBI-ID: 12098 Sci species name: Human hepatitis A virus Hu/Australia/HM175/1976 Virus type: VIRION / Virus isolate: STRAIN / Virus enveloped: No / Virus empty: No |
|---|
-Macromolecule #1: VP1
| Macromolecule | Name: VP1 / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Human hepatitis A virus Hu/Australia/HM175/1976 Human hepatitis A virus Hu/Australia/HM175/1976 |
| Molecular weight | Theoretical: 30.820629 KDa |
| Sequence | String: VGDDSGGFST TVSTEQNVPD PQVGITTMRD LKGKANRGKM DVSGVQAPVG AITTIEDPVL AKKVPETFPE LKPGESRHTS DHMSIYKFM GRSHFLCTFT FNSNNKEYTF PITLSSTSNP PHGLPSTLRW FFNLFQLYRG PLDLTIIITG ATDVDGMAWF T PVGLAVDT ...String: VGDDSGGFST TVSTEQNVPD PQVGITTMRD LKGKANRGKM DVSGVQAPVG AITTIEDPVL AKKVPETFPE LKPGESRHTS DHMSIYKFM GRSHFLCTFT FNSNNKEYTF PITLSSTSNP PHGLPSTLRW FFNLFQLYRG PLDLTIIITG ATDVDGMAWF T PVGLAVDT PWVEKESALQ IDYKTALGAV RFNTRRTGNI QIRLPWYSYL YAVSGALDGL GDKTDSTFGL VSIQIANYNH SD EYLSFSC YLSVTEQSEF YFPRAPLNSN AMLSTESMMS R |
-Macromolecule #2: VP2
| Macromolecule | Name: VP2 / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Human hepatitis A virus Hu/Australia/HM175/1976 Human hepatitis A virus Hu/Australia/HM175/1976 |
| Molecular weight | Theoretical: 24.898172 KDa |
| Sequence | String: DIEEEQMIQS VDRTAVTGAS YFTSVDQSSV HTAEVGSHQI EPLKTSVDKP GSKKTQGEKF FLIHSARWLT THALFHEVAK LDVVKLLYN EQFAVQGLLR YHTYARFGIE IQVQINPTPF QQGGLICAMV PGDQSYGSIA SLTVYPHGLL NCNINNVVRI K VPFIYTRG ...String: DIEEEQMIQS VDRTAVTGAS YFTSVDQSSV HTAEVGSHQI EPLKTSVDKP GSKKTQGEKF FLIHSARWLT THALFHEVAK LDVVKLLYN EQFAVQGLLR YHTYARFGIE IQVQINPTPF QQGGLICAMV PGDQSYGSIA SLTVYPHGLL NCNINNVVRI K VPFIYTRG AYHFKDPQYP VWELTIRVWS ELNIGTGTSA YTSLNVLARF TDLELHGLTP LSTQ |
-Macromolecule #3: VP3
| Macromolecule | Name: VP3 / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Human hepatitis A virus Hu/Australia/HM175/1976 Human hepatitis A virus Hu/Australia/HM175/1976 |
| Molecular weight | Theoretical: 27.835693 KDa |
| Sequence | String: MMRNETRVST TENVVNLSNY EDARAKMSFA LDQEDWKSDP SQGGGIKITH FTTWTSIPTL AAQFPFNASD SVGQQIKVIP VDPYFFQMT NTNPDQKCIT ALASICQMFC FWRGDLVFDF QVFPTKYHSG RLLFCFVPGN ELIDVTGITL KQATTAPCAV M DIAGVQST ...String: MMRNETRVST TENVVNLSNY EDARAKMSFA LDQEDWKSDP SQGGGIKITH FTTWTSIPTL AAQFPFNASD SVGQQIKVIP VDPYFFQMT NTNPDQKCIT ALASICQMFC FWRGDLVFDF QVFPTKYHSG RLLFCFVPGN ELIDVTGITL KQATTAPCAV M DIAGVQST LRFRVPWISD TPYRVNRYTK EAHQKGEYTA IGKLIVYCYN RLTSPSNVAH HVRVNVYLSA INLECFAPLY HA MDVTTQ |
-Macromolecule #4: FAB Heavy Chain
| Macromolecule | Name: FAB Heavy Chain / type: protein_or_peptide / ID: 4 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 23.939873 KDa |
| Sequence | String: EVKLVESGGG LVKPGGSLKL SCAASLFTHN NYGMSWVRQT PEKRLEWVAT INSTASYTYY PDSVKGRFTI SRDNAKNTLY LQMSSLRSG DTAIYYCARK NDTFSDYYFD YWGQGTTLTV SSPKTTPPSV YPLAPASAST AASMVTLGCL VKGYFPEPVT V TWNSGSLS ...String: EVKLVESGGG LVKPGGSLKL SCAASLFTHN NYGMSWVRQT PEKRLEWVAT INSTASYTYY PDSVKGRFTI SRDNAKNTLY LQMSSLRSG DTAIYYCARK NDTFSDYYFD YWGQGTTLTV SSPKTTPPSV YPLAPASAST AASMVTLGCL VKGYFPEPVT V TWNSGSLS SGVHTFPAVL QSDLYTLSSS VTVPSSTWPS ETVTCNVAHP ASSTKVDKKI VPR |
-Macromolecule #5: FAB Light Chain
| Macromolecule | Name: FAB Light Chain / type: protein_or_peptide / ID: 5 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 23.592875 KDa |
| Sequence | String: DIVLTQSPAI MSASPGERVT MTCSAHVSTD YMHWYQQKSG TSPKRWIYDT SKLASTVPDR FSGSGSGTSY SLTISSMEAE DAATYYCQQ WNNNAYTYGG GTKLEIKRAD AAPTVSIFPP SSEQLTSGGA SVVCFLNNFY PKDINVKWKI DGSERQNGVL N SWTDQDSK ...String: DIVLTQSPAI MSASPGERVT MTCSAHVSTD YMHWYQQKSG TSPKRWIYDT SKLASTVPDR FSGSGSGTSY SLTISSMEAE DAATYYCQQ WNNNAYTYGG GTKLEIKRAD AAPTVSIFPP SSEQLTSGGA SVVCFLNNFY PKDINVKWKI DGSERQNGVL N SWTDQDSK DSTYSMSSTL TLTKDEYERH NSYTCEATHK TSTSPIVKSF NRNEC |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 BASE (4k x 4k) / Average electron dose: 1.3 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller













 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)






















