+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Extended RPA-DNA nucleoprotein filament | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | DNA replication / single strand DNA-binding protein / RPA / DNA BINDING PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationnucleic acid binding / chromosome, telomeric region / DNA binding / metal ion binding Similarity search - Function | |||||||||
| Biological species |   Pyrococcus abyssi (archaea) / Pyrococcus abyssi (archaea) /  | |||||||||
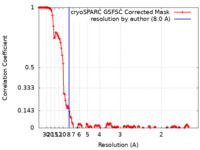
| Method | single particle reconstruction / cryo EM / Resolution: 8.0 Å | |||||||||
 Authors Authors | Madru C / Martinez-Carranza M / Sauguet L | |||||||||
| Funding support |  France, 1 items France, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2023 Journal: Nat Commun / Year: 2023Title: DNA-binding mechanism and evolution of replication protein A. Authors: Clément Madru / Markel Martínez-Carranza / Sébastien Laurent / Alessandra C Alberti / Maelenn Chevreuil / Bertrand Raynal / Ahmed Haouz / Rémy A Le Meur / Marc Delarue / Ghislaine ...Authors: Clément Madru / Markel Martínez-Carranza / Sébastien Laurent / Alessandra C Alberti / Maelenn Chevreuil / Bertrand Raynal / Ahmed Haouz / Rémy A Le Meur / Marc Delarue / Ghislaine Henneke / Didier Flament / Mart Krupovic / Pierre Legrand / Ludovic Sauguet /  Abstract: Replication Protein A (RPA) is a heterotrimeric single stranded DNA-binding protein with essential roles in DNA replication, recombination and repair. Little is known about the structure of RPA in ...Replication Protein A (RPA) is a heterotrimeric single stranded DNA-binding protein with essential roles in DNA replication, recombination and repair. Little is known about the structure of RPA in Archaea, the third domain of life. By using an integrative structural, biochemical and biophysical approach, we extensively characterize RPA from Pyrococcus abyssi in the presence and absence of DNA. The obtained X-ray and cryo-EM structures reveal that the trimerization core and interactions promoting RPA clustering on ssDNA are shared between archaea and eukaryotes. However, we also identified a helical domain named AROD (Acidic Rpa1 OB-binding Domain), and showed that, in Archaea, RPA forms an unanticipated tetrameric supercomplex in the absence of DNA. The four RPA molecules clustered within the tetramer could efficiently coat and protect stretches of ssDNA created by the advancing replisome. Finally, our results provide insights into the evolution of this primordial replication factor in eukaryotes. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_16826.map.gz emd_16826.map.gz | 229.3 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-16826-v30.xml emd-16826-v30.xml emd-16826.xml emd-16826.xml | 20.1 KB 20.1 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_16826_fsc.xml emd_16826_fsc.xml | 13.4 KB | Display |  FSC data file FSC data file |
| Images |  emd_16826.png emd_16826.png | 36.2 KB | ||
| Masks |  emd_16826_msk_1.map emd_16826_msk_1.map | 244.1 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-16826.cif.gz emd-16826.cif.gz | 6.2 KB | ||
| Others |  emd_16826_half_map_1.map.gz emd_16826_half_map_1.map.gz emd_16826_half_map_2.map.gz emd_16826_half_map_2.map.gz | 226.9 MB 226.9 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-16826 http://ftp.pdbj.org/pub/emdb/structures/EMD-16826 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-16826 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-16826 | HTTPS FTP |
-Validation report
| Summary document |  emd_16826_validation.pdf.gz emd_16826_validation.pdf.gz | 897.2 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_16826_full_validation.pdf.gz emd_16826_full_validation.pdf.gz | 896.7 KB | Display | |
| Data in XML |  emd_16826_validation.xml.gz emd_16826_validation.xml.gz | 22.3 KB | Display | |
| Data in CIF |  emd_16826_validation.cif.gz emd_16826_validation.cif.gz | 28.8 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-16826 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-16826 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-16826 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-16826 | HTTPS FTP |
-Related structure data
| Related structure data |  8oejMC  8aa9C  8aajC  8aasC  8c5yC  8c5zC  8oelC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_16826.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_16826.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.76 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_16826_msk_1.map emd_16826_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_16826_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_16826_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : RPA bound to a 100-mer poly-dT ssDNA
| Entire | Name: RPA bound to a 100-mer poly-dT ssDNA |
|---|---|
| Components |
|
-Supramolecule #1: RPA bound to a 100-mer poly-dT ssDNA
| Supramolecule | Name: RPA bound to a 100-mer poly-dT ssDNA / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#4 |
|---|---|
| Molecular weight | Theoretical: 180 KDa |
-Supramolecule #2: RPA 1,2,3
| Supramolecule | Name: RPA 1,2,3 / type: complex / ID: 2 / Parent: 1 / Macromolecule list: #1-#3 |
|---|---|
| Source (natural) | Organism:   Pyrococcus abyssi (archaea) Pyrococcus abyssi (archaea) |
-Supramolecule #3: poly dT
| Supramolecule | Name: poly dT / type: complex / ID: 3 / Parent: 1 / Macromolecule list: #4 |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: Replication factor A
| Macromolecule | Name: Replication factor A / type: protein_or_peptide / ID: 1 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Pyrococcus abyssi (archaea) Pyrococcus abyssi (archaea) |
| Molecular weight | Theoretical: 41.008965 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MSVLTKDRII EIIERKTGMS REEIEEEIRK IMEEDPYLSE QGAAALLAER LGIDLIEKEE VSLMRISELY PGMDPREVNV VGRVLKKYP PREYTRKDGS VGRVASLIIY DDSGRARVVL WDAKVSEYYN KIEVGDVIKV LDAQVKESLS GLPELHINFR A RIILNPDD ...String: MSVLTKDRII EIIERKTGMS REEIEEEIRK IMEEDPYLSE QGAAALLAER LGIDLIEKEE VSLMRISELY PGMDPREVNV VGRVLKKYP PREYTRKDGS VGRVASLIIY DDSGRARVVL WDAKVSEYYN KIEVGDVIKV LDAQVKESLS GLPELHINFR A RIILNPDD PRVEMIPPLE EVRVATYTRK KIKDIEAGDR FVEVRGTIAK VYRVLTYDAC PECKKKVDYD EGLGVWICPE HG EVQPIKM TILDFGLDDG TGYIRVTLFG DDAEELLGVS PEEIAEKIKE LEESGLTTKE AARKLAEDEF YNIIGREIVV RGN VIEDRF LGLILRASSW EDVDYRREIE RIKEELEKLG VM UniProtKB: Replication factor A |
-Macromolecule #2: RPA32 subunit of the hetero-oligomeric complex involved in homolo...
| Macromolecule | Name: RPA32 subunit of the hetero-oligomeric complex involved in homologous recombination type: protein_or_peptide / ID: 2 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Pyrococcus abyssi (archaea) Pyrococcus abyssi (archaea) |
| Molecular weight | Theoretical: 31.489309 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MSKKRMPATR LYIKDILEGY FVKSEGDFEP NYLITKYARK VYRAKIVGTV VREPLIAEDE TYGKFQVDDG TGVIWVLGFR DDTKFAKLV RKGDLVQVIG KIAEWRDDKQ ILVEGVSKVH PNMWILHRYE TLKEKIEHIK KAKIALEIYN QYGITAKSKV I AKNKGIEE ...String: MSKKRMPATR LYIKDILEGY FVKSEGDFEP NYLITKYARK VYRAKIVGTV VREPLIAEDE TYGKFQVDDG TGVIWVLGFR DDTKFAKLV RKGDLVQVIG KIAEWRDDKQ ILVEGVSKVH PNMWILHRYE TLKEKIEHIK KAKIALEIYN QYGITAKSKV I AKNKGIEE ELLEVIDELY GIMMEERSIE EPMEELLEEE IPEEKEENEL LEKAKEDILN ILRQKRTAIS RKYILKKLGD KY DEETIDD AITELLAQGE IYEPETGYYK LL UniProtKB: RPA32 subunit of the hetero-oligomeric complex involved in homologous recombination |
-Macromolecule #3: RPA14 subunit of the hetero-oligomeric complex involved in homolo...
| Macromolecule | Name: RPA14 subunit of the hetero-oligomeric complex involved in homologous recombination type: protein_or_peptide / ID: 3 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Pyrococcus abyssi (archaea) Pyrococcus abyssi (archaea) |
| Molecular weight | Theoretical: 14.008925 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: GTGDGSEVQV RRRKPAVERK ISEIREEDTR VSLIGRVIKV DKMDYMFWLD DGTGVAIIES ESDLPKVGQV VRVIGRIIRN EEGIHIYAE VIQDFSDADL EALEEIRELE RKLLPRLEGE IVW UniProtKB: RPA14 subunit of the hetero-oligomeric complex involved in homologous recombination |
-Macromolecule #4: poly dT
| Macromolecule | Name: poly dT / type: dna / ID: 4 / Number of copies: 1 / Classification: DNA |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 30.374275 KDa |
| Sequence | String: (DT)(DT)(DT)(DT)(DT)(DT)(DT)(DT)(DT)(DT) (DT)(DT)(DT)(DT)(DT)(DT)(DT)(DT)(DT)(DT) (DT)(DT)(DT)(DT)(DT)(DT)(DT)(DT)(DT) (DT)(DT)(DT)(DT)(DT)(DT)(DT)(DT)(DT)(DT) (DT) (DT)(DT)(DT)(DT)(DT)(DT) ...String: (DT)(DT)(DT)(DT)(DT)(DT)(DT)(DT)(DT)(DT) (DT)(DT)(DT)(DT)(DT)(DT)(DT)(DT)(DT)(DT) (DT)(DT)(DT)(DT)(DT)(DT)(DT)(DT)(DT) (DT)(DT)(DT)(DT)(DT)(DT)(DT)(DT)(DT)(DT) (DT) (DT)(DT)(DT)(DT)(DT)(DT)(DT)(DT) (DT)(DT)(DT)(DT)(DT)(DT)(DT)(DT)(DT)(DT) (DT)(DT) (DT)(DT)(DT)(DT)(DT)(DT)(DT) (DT)(DT)(DT)(DT)(DT)(DT)(DT)(DT)(DT)(DT) (DT)(DT)(DT) (DT)(DT)(DT)(DT)(DT)(DT) (DT)(DT)(DT)(DT)(DT)(DT)(DT)(DT)(DT)(DT) (DT)(DT)(DT)(DT) |
-Macromolecule #5: ZINC ION
| Macromolecule | Name: ZINC ION / type: ligand / ID: 5 / Number of copies: 2 / Formula: ZN |
|---|---|
| Molecular weight | Theoretical: 65.409 Da |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 8 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | TFS GLACIOS |
|---|---|
| Image recording | Film or detector model: FEI FALCON IV (4k x 4k) / Average electron dose: 40.0 e/Å2 |
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: SPOT SCAN / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.8000000000000003 µm / Nominal defocus min: 0.8 µm |
 Movie
Movie Controller
Controller










 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)