+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | RPA tetrameric supercomplex from Pyrococcus abyssi | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | DNA replication / single strand DNA-binding protein / RPA / DNA BINDING PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationresponse to ionizing radiation / double-strand break repair via homologous recombination / nucleic acid binding / DNA binding / metal ion binding Similarity search - Function | |||||||||
| Biological species |   Pyrococcus abyssi (archaea) Pyrococcus abyssi (archaea) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.35 Å | |||||||||
 Authors Authors | Madru C / Martinez-Carranza M / Legrand P / Sauguet L | |||||||||
| Funding support |  France, 1 items France, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2023 Journal: Nat Commun / Year: 2023Title: DNA-binding mechanism and evolution of replication protein A. Authors: Clément Madru / Markel Martínez-Carranza / Sébastien Laurent / Alessandra C Alberti / Maelenn Chevreuil / Bertrand Raynal / Ahmed Haouz / Rémy A Le Meur / Marc Delarue / Ghislaine ...Authors: Clément Madru / Markel Martínez-Carranza / Sébastien Laurent / Alessandra C Alberti / Maelenn Chevreuil / Bertrand Raynal / Ahmed Haouz / Rémy A Le Meur / Marc Delarue / Ghislaine Henneke / Didier Flament / Mart Krupovic / Pierre Legrand / Ludovic Sauguet /  Abstract: Replication Protein A (RPA) is a heterotrimeric single stranded DNA-binding protein with essential roles in DNA replication, recombination and repair. Little is known about the structure of RPA in ...Replication Protein A (RPA) is a heterotrimeric single stranded DNA-binding protein with essential roles in DNA replication, recombination and repair. Little is known about the structure of RPA in Archaea, the third domain of life. By using an integrative structural, biochemical and biophysical approach, we extensively characterize RPA from Pyrococcus abyssi in the presence and absence of DNA. The obtained X-ray and cryo-EM structures reveal that the trimerization core and interactions promoting RPA clustering on ssDNA are shared between archaea and eukaryotes. However, we also identified a helical domain named AROD (Acidic Rpa1 OB-binding Domain), and showed that, in Archaea, RPA forms an unanticipated tetrameric supercomplex in the absence of DNA. The four RPA molecules clustered within the tetramer could efficiently coat and protect stretches of ssDNA created by the advancing replisome. Finally, our results provide insights into the evolution of this primordial replication factor in eukaryotes. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_16444.map.gz emd_16444.map.gz | 203.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-16444-v30.xml emd-16444-v30.xml emd-16444.xml emd-16444.xml | 18.3 KB 18.3 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_16444_fsc.xml emd_16444_fsc.xml | 12.7 KB | Display |  FSC data file FSC data file |
| Images |  emd_16444.png emd_16444.png | 83 KB | ||
| Masks |  emd_16444_msk_1.map emd_16444_msk_1.map | 216 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-16444.cif.gz emd-16444.cif.gz | 5.8 KB | ||
| Others |  emd_16444_half_map_1.map.gz emd_16444_half_map_1.map.gz emd_16444_half_map_2.map.gz emd_16444_half_map_2.map.gz | 200 MB 200 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-16444 http://ftp.pdbj.org/pub/emdb/structures/EMD-16444 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-16444 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-16444 | HTTPS FTP |
-Related structure data
| Related structure data |  8c5yMC  8aa9C  8aajC  8aasC  8c5zC  8oejC  8oelC  15300 M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_16444.map.gz / Format: CCP4 / Size: 216 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_16444.map.gz / Format: CCP4 / Size: 216 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
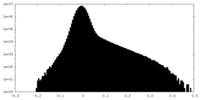
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.86 Å | ||||||||||||||||||||||||||||||||||||
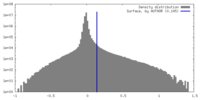
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_16444_msk_1.map emd_16444_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
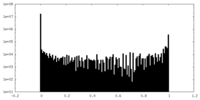
| Density Histograms |
-Half map: #1
| File | emd_16444_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_16444_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : RPA tetrameric supercomplex
| Entire | Name: RPA tetrameric supercomplex |
|---|---|
| Components |
|
-Supramolecule #1: RPA tetrameric supercomplex
| Supramolecule | Name: RPA tetrameric supercomplex / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#3 |
|---|---|
| Source (natural) | Organism:   Pyrococcus abyssi (archaea) Pyrococcus abyssi (archaea) |
| Molecular weight | Theoretical: 345 KDa |
-Macromolecule #1: Replication factor A
| Macromolecule | Name: Replication factor A / type: protein_or_peptide / ID: 1 / Number of copies: 4 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Pyrococcus abyssi (archaea) / Strain: GE5 / ORSAY Pyrococcus abyssi (archaea) / Strain: GE5 / ORSAY |
| Molecular weight | Theoretical: 20.234057 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: VATYTRKKIK DIEAGDRFVE VRGTIAKVYR VLTYDACPEC KKKVDYDEGL GVWICPEHGE VQPIKMTILD FGLDDGTGYI RVTLFGDDA EELLGVSPEE IAEKIKELEE SGLTTKEAAR KLAEDEFYNI IGREIVVRGN VIEDRFLGLI LRASSWEDVD Y RREIERIK EELEKLGVM UniProtKB: Replication factor A |
-Macromolecule #2: RPA32 subunit of the hetero-oligomeric complex involved in homolo...
| Macromolecule | Name: RPA32 subunit of the hetero-oligomeric complex involved in homologous recombination type: protein_or_peptide / ID: 2 / Number of copies: 4 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Pyrococcus abyssi (archaea) / Strain: GE5 / ORSAY Pyrococcus abyssi (archaea) / Strain: GE5 / ORSAY |
| Molecular weight | Theoretical: 20.930475 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: KKRMPATRLY IKDILEGYFV KSEGDFEPNY LITKYARKVY RAKIVGTVVR EPLIAEDETY GKFQVDDGTG VIWVLGFRDD TKFAKLVRK GDLVQVIGKI AEWRDDKQIL VEGVSKVHPN MWILHRYETL KEKIEHIKKA KIALEIYNQY GITAKSKVIA K NKGIEEEL LEVIDELYGI MM UniProtKB: RPA32 subunit of the hetero-oligomeric complex involved in homologous recombination |
-Macromolecule #3: RPA14 subunit of the hetero-oligomeric complex involved in homolo...
| Macromolecule | Name: RPA14 subunit of the hetero-oligomeric complex involved in homologous recombination type: protein_or_peptide / ID: 3 / Number of copies: 4 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Pyrococcus abyssi (archaea) / Strain: GE5 / ORSAY Pyrococcus abyssi (archaea) / Strain: GE5 / ORSAY |
| Molecular weight | Theoretical: 13.078994 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: RRRKPAVERK ISEIREEDTR VSLIGRVIKV DKMDYMFWLD DGTGVAIIES ESDLPKVGQV VRVIGRIIRN EEGIHIYAEV IQDFSDADL EALEEIRELE RKLLPRLEGE IVW UniProtKB: RPA14 subunit of the hetero-oligomeric complex involved in homologous recombination |
-Macromolecule #4: ZINC ION
| Macromolecule | Name: ZINC ION / type: ligand / ID: 4 / Number of copies: 4 / Formula: ZN |
|---|---|
| Molecular weight | Theoretical: 65.409 Da |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 8 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 40.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: SPOT SCAN / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.8000000000000003 µm / Nominal defocus min: 0.8 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller










 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)