[English] 日本語
 Yorodumi
Yorodumi- EMDB-12614: XPB-containing part of TFIIH in a post-translocated state (with A... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-12614 | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | XPB-containing part of TFIIH in a post-translocated state (with ADP-BeF3) | ||||||||||||||||||
 Map data Map data | Local resolution filtered and sharpened map | ||||||||||||||||||
 Sample Sample |
| ||||||||||||||||||
 Keywords Keywords | Initiation / TRANSCRIPTION | ||||||||||||||||||
| Function / homology |  Function and homology information Function and homology informationcore TFIIH complex portion of holo TFIIH complex / nucleotide-excision repair, DNA duplex unwinding / hair cell differentiation / nucleotide-excision repair factor 3 complex / transcription factor TFIIE complex / nucleotide-excision repair, preincision complex assembly / UV protection / transcription open complex formation at RNA polymerase II promoter / transcription factor TFIIH holo complex / transcription factor TFIIH core complex ...core TFIIH complex portion of holo TFIIH complex / nucleotide-excision repair, DNA duplex unwinding / hair cell differentiation / nucleotide-excision repair factor 3 complex / transcription factor TFIIE complex / nucleotide-excision repair, preincision complex assembly / UV protection / transcription open complex formation at RNA polymerase II promoter / transcription factor TFIIH holo complex / transcription factor TFIIH core complex / DNA 3'-5' helicase / RNA Polymerase I Transcription Termination / transcription preinitiation complex / regulation of mitotic cell cycle phase transition / 3'-5' DNA helicase activity / RNA Pol II CTD phosphorylation and interaction with CE during HIV infection / RNA Pol II CTD phosphorylation and interaction with CE / transcription factor TFIID complex / Formation of the Early Elongation Complex / Formation of the HIV-1 Early Elongation Complex / RNA polymerase II general transcription initiation factor activity / mRNA Capping / HIV Transcription Initiation / RNA Polymerase II HIV Promoter Escape / Transcription of the HIV genome / RNA Polymerase II Promoter Escape / RNA Polymerase II Transcription Pre-Initiation And Promoter Opening / RNA Polymerase II Transcription Initiation / RNA Polymerase II Transcription Initiation And Promoter Clearance / RNA Polymerase I Transcription Initiation / ATPase activator activity / DNA topological change / RNA polymerase II transcribes snRNA genes / Tat-mediated elongation of the HIV-1 transcript / embryonic organ development / transcription elongation by RNA polymerase I / Formation of HIV-1 elongation complex containing HIV-1 Tat / transcription-coupled nucleotide-excision repair / Formation of HIV elongation complex in the absence of HIV Tat / RNA Polymerase II Transcription Elongation / Formation of RNA Pol II elongation complex / response to UV / RNA Polymerase II Pre-transcription Events / maturation of SSU-rRNA from tricistronic rRNA transcript (SSU-rRNA, 5.8S rRNA, LSU-rRNA) / isomerase activity / promoter-specific chromatin binding / TP53 Regulates Transcription of DNA Repair Genes / nucleotide-excision repair / transcription initiation at RNA polymerase II promoter / RNA Polymerase I Promoter Escape / transcription elongation by RNA polymerase II / NoRC negatively regulates rRNA expression / Transcription-Coupled Nucleotide Excision Repair (TC-NER) / cellular response to gamma radiation / Formation of TC-NER Pre-Incision Complex / Dual Incision in GG-NER / Formation of Incision Complex in GG-NER / Dual incision in TC-NER / Gap-filling DNA repair synthesis and ligation in TC-NER / protein localization / double-stranded DNA binding / response to oxidative stress / transcription by RNA polymerase II / damaged DNA binding / response to hypoxia / nuclear speck / positive regulation of apoptotic process / DNA repair / nucleolus / apoptotic process / ATP hydrolysis activity / DNA binding / nucleoplasm / ATP binding / nucleus / metal ion binding / cytosol / cytoplasm Similarity search - Function | ||||||||||||||||||
| Biological species |  Homo sapiens (human) / Homo sapiens (human) /  Human mastadenovirus C Human mastadenovirus C | ||||||||||||||||||
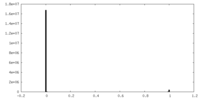
| Method | single particle reconstruction / cryo EM / Resolution: 2.9 Å | ||||||||||||||||||
 Authors Authors | Aibara S / Schilbach S | ||||||||||||||||||
| Funding support |  Germany, 5 items Germany, 5 items
| ||||||||||||||||||
 Citation Citation |  Journal: Nature / Year: 2021 Journal: Nature / Year: 2021Title: Structures of mammalian RNA polymerase II pre-initiation complexes. Authors: Shintaro Aibara / Sandra Schilbach / Patrick Cramer /  Abstract: The initiation of transcription is a focal point for the regulation of gene activity during mammalian cell differentiation and development. To initiate transcription, RNA polymerase II (Pol II) ...The initiation of transcription is a focal point for the regulation of gene activity during mammalian cell differentiation and development. To initiate transcription, RNA polymerase II (Pol II) assembles with general transcription factors into a pre-initiation complex (PIC) that opens promoter DNA. Previous work provided the molecular architecture of the yeast and human PIC and a topological model for DNA opening by the general transcription factor TFIIH. Here we report the high-resolution cryo-electron microscopy structure of PIC comprising human general factors and Sus scrofa domesticus Pol II, which is 99.9% identical to human Pol II. We determine the structures of PIC with closed and opened promoter DNA at 2.5-2.8 Å resolution, and resolve the structure of TFIIH at 2.9-4.0 Å resolution. We capture the TFIIH translocase XPB in the pre- and post-translocation states, and show that XPB induces and propagates a DNA twist to initiate the opening of DNA approximately 30 base pairs downstream of the TATA box. We also provide evidence that DNA opening occurs in two steps and leads to the detachment of TFIIH from the core PIC, which may stop DNA twisting and enable RNA chain initiation. | ||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_12614.map.gz emd_12614.map.gz | 285.2 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-12614-v30.xml emd-12614-v30.xml emd-12614.xml emd-12614.xml | 27 KB 27 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_12614_fsc.xml emd_12614_fsc.xml | 15.9 KB | Display |  FSC data file FSC data file |
| Images |  emd_12614.png emd_12614.png | 151.1 KB | ||
| Masks |  emd_12614_msk_1.map emd_12614_msk_1.map | 347.6 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-12614.cif.gz emd-12614.cif.gz | 7.3 KB | ||
| Others |  emd_12614_additional_1.map.gz emd_12614_additional_1.map.gz emd_12614_half_map_1.map.gz emd_12614_half_map_1.map.gz emd_12614_half_map_2.map.gz emd_12614_half_map_2.map.gz | 203.4 MB 278.5 MB 278.2 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-12614 http://ftp.pdbj.org/pub/emdb/structures/EMD-12614 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-12614 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-12614 | HTTPS FTP |
-Validation report
| Summary document |  emd_12614_validation.pdf.gz emd_12614_validation.pdf.gz | 601.9 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_12614_full_validation.pdf.gz emd_12614_full_validation.pdf.gz | 601.4 KB | Display | |
| Data in XML |  emd_12614_validation.xml.gz emd_12614_validation.xml.gz | 24 KB | Display | |
| Data in CIF |  emd_12614_validation.cif.gz emd_12614_validation.cif.gz | 32.3 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-12614 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-12614 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-12614 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-12614 | HTTPS FTP |
-Related structure data
| Related structure data |  7nvvMC  7nvrC  7nvsC  7nvtC  7nvuC  7nvwC  7nvxC  7nvyC  7nvzC  7nw0C C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_12614.map.gz / Format: CCP4 / Size: 347.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_12614.map.gz / Format: CCP4 / Size: 347.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Local resolution filtered and sharpened map | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.05 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Mask #1
| File |  emd_12614_msk_1.map emd_12614_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: Local resolution filtered map, unsharpened
| File | emd_12614_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Local resolution filtered map, unsharpened | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Unfiltered half map 1
| File | emd_12614_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Unfiltered half map 1 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Unfiltered half map 2
| File | emd_12614_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Unfiltered half map 2 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
+Entire : XPB-containing part of TFIIH
+Supramolecule #1: XPB-containing part of TFIIH
+Macromolecule #1: General transcription factor IIH subunit 4
+Macromolecule #2: General transcription factor IIH subunit 5
+Macromolecule #3: General transcription and DNA repair factor IIH helicase subunit XPB
+Macromolecule #6: General transcription factor IIE subunit 1
+Macromolecule #7: Unassigned peptide, likely XPB
+Macromolecule #8: Unassigned Peptide, likely TFIIE-Beta
+Macromolecule #4: Non-template DNA
+Macromolecule #5: Template DNA
+Macromolecule #9: ADENOSINE-5'-DIPHOSPHATE
+Macromolecule #10: MAGNESIUM ION
+Macromolecule #11: BERYLLIUM TRIFLUORIDE ION
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 43.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller







































 X (Sec.)
X (Sec.) Y (Row.)
Y (Row.) Z (Col.)
Z (Col.)













































 Trichoplusia ni (cabbage looper)
Trichoplusia ni (cabbage looper)