+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-10532 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of FANCD2 in complex with FANCI | |||||||||
 Map data Map data | Post processed map from Relion | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Fanconi anaemia / ubiquitin / DNA repair / DNA damage / inter-strand crosslink / DNA BINDING PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationFanconi Anemia Pathway in DNA repair / Fanconi Anemia Pathway / double-strand break repair involved in meiotic recombination / homologous chromosome pairing at meiosis / mitotic intra-S DNA damage checkpoint signaling / interstrand cross-link repair / condensed chromosome / DNA polymerase binding / DNA repair / nucleoplasm / nucleus Similarity search - Function | |||||||||
| Biological species |  | |||||||||
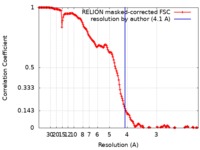
| Method | single particle reconstruction / cryo EM / Resolution: 4.1 Å | |||||||||
 Authors Authors | Alcon P / Shakeel S | |||||||||
| Funding support |  United Kingdom, 2 items United Kingdom, 2 items
| |||||||||
 Citation Citation |  Journal: Nat Struct Mol Biol / Year: 2020 Journal: Nat Struct Mol Biol / Year: 2020Title: FANCD2-FANCI is a clamp stabilized on DNA by monoubiquitination of FANCD2 during DNA repair. Authors: Pablo Alcón / Shabih Shakeel / Zhuo A Chen / Juri Rappsilber / Ketan J Patel / Lori A Passmore /   Abstract: Vertebrate DNA crosslink repair excises toxic replication-blocking DNA crosslinks. Numerous factors involved in crosslink repair have been identified, and mutations in their corresponding genes cause ...Vertebrate DNA crosslink repair excises toxic replication-blocking DNA crosslinks. Numerous factors involved in crosslink repair have been identified, and mutations in their corresponding genes cause Fanconi anemia (FA). A key step in crosslink repair is monoubiquitination of the FANCD2-FANCI heterodimer, which then recruits nucleases to remove the DNA lesion. Here, we use cryo-EM to determine the structures of recombinant chicken FANCD2 and FANCI complexes. FANCD2-FANCI adopts a closed conformation when the FANCD2 subunit is monoubiquitinated, creating a channel that encloses double-stranded DNA (dsDNA). Ubiquitin is positioned at the interface of FANCD2 and FANCI, where it acts as a covalent molecular pin to trap the complex on DNA. In contrast, isolated FANCD2 is a homodimer that is unable to bind DNA, suggestive of an autoinhibitory mechanism that prevents premature activation. Together, our work suggests that FANCD2-FANCI is a clamp that is locked onto DNA by ubiquitin, with distinct interfaces that may recruit other DNA repair factors. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_10532.map.gz emd_10532.map.gz | 18.3 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-10532-v30.xml emd-10532-v30.xml emd-10532.xml emd-10532.xml | 22.6 KB 22.6 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_10532_fsc.xml emd_10532_fsc.xml | 15.9 KB | Display |  FSC data file FSC data file |
| Images |  emd_10532.png emd_10532.png | 52.2 KB | ||
| Filedesc metadata |  emd-10532.cif.gz emd-10532.cif.gz | 7.6 KB | ||
| Others |  emd_10532_additional.map.gz emd_10532_additional.map.gz emd_10532_half_map_1.map.gz emd_10532_half_map_1.map.gz emd_10532_half_map_2.map.gz emd_10532_half_map_2.map.gz | 277.9 MB 278.1 MB 278.6 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-10532 http://ftp.pdbj.org/pub/emdb/structures/EMD-10532 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-10532 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-10532 | HTTPS FTP |
-Validation report
| Summary document |  emd_10532_validation.pdf.gz emd_10532_validation.pdf.gz | 742.2 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_10532_full_validation.pdf.gz emd_10532_full_validation.pdf.gz | 741.7 KB | Display | |
| Data in XML |  emd_10532_validation.xml.gz emd_10532_validation.xml.gz | 22 KB | Display | |
| Data in CIF |  emd_10532_validation.cif.gz emd_10532_validation.cif.gz | 28.3 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-10532 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-10532 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-10532 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-10532 | HTTPS FTP |
-Related structure data
| Related structure data |  6tngMC  6tnfC  6tniC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | |
| EM raw data |  EMPIAR-10609 (Title: Unaligned movies for FANCD2-FANCI + DNA / Data size: 1.7 TB / Data #1: Unaligned movies [micrographs - multiframe]) EMPIAR-10609 (Title: Unaligned movies for FANCD2-FANCI + DNA / Data size: 1.7 TB / Data #1: Unaligned movies [micrographs - multiframe]) |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_10532.map.gz / Format: CCP4 / Size: 347.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_10532.map.gz / Format: CCP4 / Size: 347.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Post processed map from Relion | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.11 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Additional map: Map from auto-refinement in Relion
| File | emd_10532_additional.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Map from auto-refinement in Relion | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half2 map from auto-refinement in Relion
| File | emd_10532_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half2 map from auto-refinement in Relion | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half1 map from auto-refinement in Relion
| File | emd_10532_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half1 map from auto-refinement in Relion | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Complex of monoubiquitinated FANCD2 and FANCI
| Entire | Name: Complex of monoubiquitinated FANCD2 and FANCI |
|---|---|
| Components |
|
-Supramolecule #1: Complex of monoubiquitinated FANCD2 and FANCI
| Supramolecule | Name: Complex of monoubiquitinated FANCD2 and FANCI / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 300 KDa |
-Macromolecule #1: Uncharacterized protein
| Macromolecule | Name: Uncharacterized protein / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 160.932328 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MVSKRKLSKI DAAEESSKTD LQSRCPETKR SRISDKRAPS QGGLENEGVF EELLRTSGII LKVGEGQNEI AVDQTAFQKK LRVALEKHP SYPGVVNEFI SGLESHIKDR SQFKNCLLPC TPARTEGSRT LVHSYCESLI KLLLGIKILQ PAVVTLLLEK I PEFFFDVV ...String: MVSKRKLSKI DAAEESSKTD LQSRCPETKR SRISDKRAPS QGGLENEGVF EELLRTSGII LKVGEGQNEI AVDQTAFQKK LRVALEKHP SYPGVVNEFI SGLESHIKDR SQFKNCLLPC TPARTEGSRT LVHSYCESLI KLLLGIKILQ PAVVTLLLEK I PEFFFDVV GTFGTNFPRL IVNQFKWLDG LLDSQDLVKK LMQMLSVSPV PIQHDIITSL PEILEDSQQN EVARELSCLL KQ GRRLTVP ILDALSRLDL DAELLAKVRQ SAMTIVPSVK LEDLPVVIKF ILHNVKAADA VEVISDLRKS LDLSSCVLPL QLL GSQRKL KSQAQASSSM SQVTTSQNCV KLLFDVIKLA VRFQKDVSEA WIKAIENSTS VSDHKVLDLI VLLLIHSTNS KNRK QTEKV LRSKIRLGCM PEQLMQNAFQ NHSMVIKDFF PSILSLAQTF LHSAHPAVVS FGSCMYKQAF AVFDSYCQQE VVCAL VTHV CSGNETELDI SLDVLTDLVI LHPSLLLRYA TFVKTILDSM QKLNPCQIRK LFYILSTLAF SQRQEGSYIQ DDMHMV IRK WLSSSVPNHK QMGIIGAVTM MGSVALKRNE ADGGLLERPE LSIECDGQLS TLLDLVGFCC EQTPEVLALY YDELANL IE KQKGNLDLQL LDKFGKSLVE DFPNDFVVDL SPTVDGSFLF PVKSLYNLDE DETQGAIAIN LLPLVSQSEP GRVADEMS N SRKRVVSPIC LSPCFRLLRL YTGEQNNGSL EEIDALLGCP LYLTDLEVEG KLDSLSKQER EFLCSLLFYA LNWFREVVN AFCQQQDAEM KGKVLTRLQN ITELQNVLGK CLAATPGYVP PPATFDSEAP EGVPSINAGG PVRKKNGKKR KSDSSKACSA ERTQADESS DGNQPDTELS ELEKSAAEKE TGNPLAQLQS YRPYFRELDL EVFSVLHCGL LTKSILDTEM HTEASEVVQL G PAELCFLL DDMCWKLEHV LTPGSTRRVP FLKERGNKDV GFSHLCQRSP KEVAVCVVKL LKPLCNHMEN MHNYFQTVIP NQ GVVDESG LNIQEYQLMS SCYHQLLLAF RLLFAWSGFS QHENSNLLRS ALQVLADRLK PGETEFLPLE ELISESFQYL LNF QASIPS FQCAFILTQV LMAISEKPMT GWKREKMASL AKQFLCQSWM KPGGDREKGS HFNSALHTLL CVYLEHTDNI LKAI EEISS VGVPELINSA KDGCSSTYPT LSRQTFPVFF RVMMAQLESS VKSIPAGKPS DSGEVQLEKL LKWNIAVRNF HILIN LVKV FDSRPVLSIC LKYGRLFVEA FLKLAMPLLD HSFKKHRDDV QSLLKTLQLS TRQLHHMCGH SKIHQDLGLT NHVPLL KKS LEQFVYRVKA MLAFNHCQEA FWVGVLKNRD LQGEEILSQA SAAPEEDSAE GSEEDTEDSA AEEPDGTDSD SGGAGR UniProtKB: Fanconi anemia complementation group D2 |
-Macromolecule #2: Fanconi anemia complementation group I
| Macromolecule | Name: Fanconi anemia complementation group I / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 149.458297 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MAQRILQLAA EGSPERLQEA LQGLTEGELG DMVTRQALRG RETAALLKGI FKGSPCSQQS GVLRRLQVYK HCVSLVESGD LHVGKVSEI IGLLMLEARQ LPGHALAELA TLFVEVIKRG SLSNGKSLEL FSTVLTALSN SKESLAYGKG ELNGEEFKKQ L INTLCSSK ...String: MAQRILQLAA EGSPERLQEA LQGLTEGELG DMVTRQALRG RETAALLKGI FKGSPCSQQS GVLRRLQVYK HCVSLVESGD LHVGKVSEI IGLLMLEARQ LPGHALAELA TLFVEVIKRG SLSNGKSLEL FSTVLTALSN SKESLAYGKG ELNGEEFKKQ L INTLCSSK WDPQCVIHLA NMFRDIPLSG EELQFVVEKV LRMFSKLDLQ EIPPLVYQLL LLSAKGSKKT VLEGIISFFN QL DKRQKEE QRVPQSADLE VATVPLDQLR HVEGTVILHI VSAINLDQDI GEELIKHLKT EQQKDPGKAL CPFSVSLLLS TAV KHRLQE QIFDFLKTSI TRSCKDLQIL QASKFLQDLC PQQYDVTAVI LEVVKNSAFG WDHVTQGLVD LGFSLMESYE PKKS FGGKA AETNLGLSKM PAQQACKLGA SILLETFKVH EPIRSDILEQ VLNRVLTKAA SPVSHFIDLL SNIVVSAPLV LQNSS SRVT ETFDNLSFLP IDTVQGLLRA VQPLLKVSMS VRDSLILVLQ KAIFSRQLDA RKAAVAGFLL LLRNFKILGS LTSSQC SQA IGATQVQADV HACYNSAANE AFCLEILGSL RRCLSQQADV RLMLYEGFYD VLRRNSQLAS SIMETLLSQI KQYYLPQ QD LLPPLKLEGC IMAQGDQIFL QEPLAHLLCC IQHCLAWYKS TVHLCKGAED EEEEEDVGFE QNFEEMLESV TRRMIKSE L EDFELDKSAD FSPSSGVGVK NNIYAIQVMG ICEVLIEYNF KIGNFSKNKF EDVLGLFTCY NKLSEILKEK AGKNKSTLG NRIARSFLSM GFVSTLLTAL FRDNAQSHEE SLAVLRSSTE FMRYAVSVAL QKVQQLEEMG QTDGPDGQNP EKMFQNLCKI TRVLLWRYT SIPTAVEESG KKKGKSISLL CLEGLLRIFN TMQQLYAARI PQFLQALDIT DGDAEEADIN VTEKAAFQIR Q FQRSLVNQ LSSAEDDFNS KETQLLITIL STLSKLLDPG SQQFLQFLTW TVKICKENAL EDLSCCKGLL TLLFSLHVLY KS PVSLLRE LAQDIHACLG DIDQDVEIES RSHFAIVNVK TAAPTVCLLV LGQADKVLEE VDWLIKRLTI LGSDTSEDST QAS NQTQAL EKGVILQLGT LLTVFHELVQ TALPAGSCVD SLLRSLSKTY AILTSLIKHY IQACRSTSNT VPGRLEKLVK LSGS HLTPQ CYSFITYVQN IHSESLSFAE EKKKKKKEDE TAVVSTVMAK VLRDTKPIPN LIFAIEQYEK FLIHLSKKSK VNLMQ YMKL STSRDFRINA SMLDSVLQEQ NTEDAENEPD NNQSGTAEQP DENQEPQKKR RRKK UniProtKB: Fanconi anemia group I protein |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.3 mg/mL |
|---|---|
| Buffer | pH: 8 Details: 50 mM HEPES, 100 mM imidazole, 150 mM NaCl, 1 mM TCEP |
| Grid | Model: UltrAuFoil / Material: GOLD / Mesh: 300 |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277.15 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Specialist optics | Energy filter - Slit width: 20 eV |
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 70.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 50.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 3.5 µm / Nominal defocus min: 1.8 µm / Nominal magnification: 81000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller














 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)