+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-12017 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
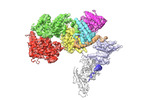
| Title | VAR2CSA full ectodomain | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | VAR2CSA / CELL ADHESION / malaria / pfEMP1 | |||||||||
| Function / homology |  Function and homology information Function and homology information | |||||||||
| Biological species |  | |||||||||
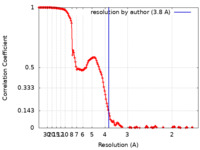
| Method | single particle reconstruction / cryo EM / Resolution: 3.8 Å | |||||||||
 Authors Authors | Wang KT / Gourdon PE | |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2021 Journal: Nat Commun / Year: 2021Title: Cryo-EM reveals the architecture of placental malaria VAR2CSA and provides molecular insight into chondroitin sulfate binding. Authors: Kaituo Wang / Robert Dagil / Thomas Lavstsen / Sandeep K Misra / Charlotte B Spliid / Yong Wang / Tobias Gustavsson / Daniel R Sandoval / Elena Ethel Vidal-Calvo / Swati Choudhary / Mette Ø ...Authors: Kaituo Wang / Robert Dagil / Thomas Lavstsen / Sandeep K Misra / Charlotte B Spliid / Yong Wang / Tobias Gustavsson / Daniel R Sandoval / Elena Ethel Vidal-Calvo / Swati Choudhary / Mette Ø Agerbaek / Kresten Lindorff-Larsen / Morten A Nielsen / Thor G Theander / Joshua S Sharp / Thomas Mandel Clausen / Pontus Gourdon / Ali Salanti /    Abstract: Placental malaria can have severe consequences for both mother and child and effective vaccines are lacking. Parasite-infected red blood cells sequester in the placenta through interaction between ...Placental malaria can have severe consequences for both mother and child and effective vaccines are lacking. Parasite-infected red blood cells sequester in the placenta through interaction between parasite-expressed protein VAR2CSA and the glycosaminoglycan chondroitin sulfate A (CS) abundantly present in the intervillous space. Here, we report cryo-EM structures of the VAR2CSA ectodomain at up to 3.1 Å resolution revealing an overall V-shaped architecture and a complex domain organization. Notably, the surface displays a single significantly electropositive patch, compatible with binding of negatively charged CS. Using molecular docking and molecular dynamics simulations as well as comparative hydroxyl radical protein foot-printing of VAR2CSA in complex with placental CS, we identify the CS-binding groove, intersecting with the positively charged patch of the central VAR2CSA structure. We identify distinctive conserved structural features upholding the macro-molecular domain complex and CS binding capacity of VAR2CSA as well as divergent elements possibly allowing immune escape at or near the CS binding site. These observations will support rational design of second-generation placental malaria vaccines. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_12017.map.gz emd_12017.map.gz | 161.3 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-12017-v30.xml emd-12017-v30.xml emd-12017.xml emd-12017.xml | 19.5 KB 19.5 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_12017_fsc.xml emd_12017_fsc.xml | 15.4 KB | Display |  FSC data file FSC data file |
| Images |  emd_12017.png emd_12017.png | 78.9 KB | ||
| Filedesc metadata |  emd-12017.cif.gz emd-12017.cif.gz | 8.1 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-12017 http://ftp.pdbj.org/pub/emdb/structures/EMD-12017 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-12017 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-12017 | HTTPS FTP |
-Related structure data
| Related structure data |  7b52MC  7b54C  7nnhC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_12017.map.gz / Format: CCP4 / Size: 325 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_12017.map.gz / Format: CCP4 / Size: 325 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.83 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : VAR2CSA full ectodomain
| Entire | Name: VAR2CSA full ectodomain |
|---|---|
| Components |
|
-Supramolecule #1: VAR2CSA full ectodomain
| Supramolecule | Name: VAR2CSA full ectodomain / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: Erythrocyte membrane protein 1
| Macromolecule | Name: Erythrocyte membrane protein 1 / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 307.549812 KDa |
| Recombinant expression | Organism:  Trichoplusia ni (cabbage looper) Trichoplusia ni (cabbage looper) |
| Sequence | String: MDSTSTIANK IEEYLGAKSD DSKIDELLKA DPSEVEYYRS GGDGDYLKNN ICKITVNHSD SGKYDPCEKK LPPYDDNDQW KCQQNSSDG SGKPENICVP PRRERLCTYN LENLKFDKIR DNNAFLADVL LTARNEGEKI VQNHPDTNSS NVCNALERSF A DLADIIRG ...String: MDSTSTIANK IEEYLGAKSD DSKIDELLKA DPSEVEYYRS GGDGDYLKNN ICKITVNHSD SGKYDPCEKK LPPYDDNDQW KCQQNSSDG SGKPENICVP PRRERLCTYN LENLKFDKIR DNNAFLADVL LTARNEGEKI VQNHPDTNSS NVCNALERSF A DLADIIRG TDQWKGTNSN LEKNLKQMFA KIRENDKVLQ DKYPKDQKYT KLREAWWNAN RQKVWEVITC GARSNDLLIK RG WRTSGKS DRKKNFELCR KCGHYEKEVP TKLDYVPQFL RWLTEWIEDF YREKQNLIDD MERHREECTR EDHKSKEGTS YCS TCKDKC KKYCECVKKW KTEWENQENK YKDLYEQNKN KTSQKNTSRY DDYVKDFFEK LEANYSSLEN YIKGDPYFAE YATK LSFIL NPSDANNPSG ETANHNDEAC NCNESGISSV GQAQTSGPSS NKTCITHSSI KTNKKKECKD VKLGVRENDK DLKIC VIED TSLSGVDNCC CQDLLGILQE NCSDNKRGSS SNDSCDNKNQ DECQKKLEKV FASLTNGYKC DKCKSGTSRS KKKWIW KKS SGNEEGLQEE YANTIGLPPR TQSLYLGNLP KLENVCEDVK DINFDTKEKF LAGCLIVSFH EGKNLKKRYP QNKNSGN KE NLCKALEYSF ADYGDLIKGT SIWDNEYTKD LELNLQNNFG KLFGKYIKKN NTAEQDTSYS SLDELRESWW NTNKKYIW T AMKHGAEMNI TTCNADGSVT GSGSSCDDIP TIDLIPQYLR FLQEWVENFC EQRQAKVKDV ITNCKSCKES GNKCKTECK TKCKDECEKY KKFIEACGTA GGGIGTAGSP WSKRWDQIYK RYSKHIEDAK RNRKAGTKNC GTSSTTNAAA STDENKCVQS DIDSFFKHL IDIGLTTPSS YLSNVLDDNI CGADKAPWTT YTTYTTTEKC NKERDKSKSQ SSDTLVVVNV PSPLGNTPYR Y KYACQCKI PTNEETCDDR KEYMNQWSCG SARTMKRGYK NDNYELCKYN GVDVKPTTVR SNSSKLDGND VTFFNLFEQW NK EIQYQIE QYMTNANISC IDEKEVLDSV SDEGTPKVRG GYEDGRNNNT DQGTNCKEKC KCYKLWIEKI NDQWGKQKDN YNK FRSKQI YDANKGSQNK KVVSLSNFLF FSCWEEYIQK YFNGDWSKIK NIGSDTFEFL IKKCGNNSAH GEEIFSEKLK NAEK KCKEN ESTDTNINKS ETSCDLNATN YIRGCQSKTY DGKIFPGKGG EKQWICKDTI IHGDTNGACI PPRTQNLCVG ELWDK SYGG RSNIKNDTKE LLKEKIKNAI HKETELLYEY HDTGTAIISK NDKKGQKGKN DPNGLPKGFC HAVQRSFIDY KNMILG TSV NIYEHIGKLQ EDIKKIIEKG TPQQKDKIGG VGSSTENVNA WWKGIEREMW DAVRCAITKI NKKNNNSIFN GDECGVS PP TGNDEDQSVS WFKEWGEQFC IERLRYEQNI REACTINGKN EKKCINSKSG QGDKIQGACK RKCEKYKKYI SEKKQEWD K QKTKYENKYV GKSASDLLKE NYPECISANF DFIFNDNIEY KTYYPYGDYS SICSCEQVKY YKYNNAEKKN NKSLCYEKD NDMTWSKKYI KKLENGRSLE GVYVPPRRQQ LCLYELFPII IKNEEGMEKA KEELLETLQI VAEREAYYLW KQYNPTGKGI DDANKKACC AIRGSFYDLE DIIKGNDLVH DEYTKYIDSK LNEIFGSSNT NDIDTKRART DWWENETITN GTDRKTIRQL V WDAMQSGV RYAVEEKNEN FPLCMGVEHI GIAKPQFIRW LEEWTNEFCE KYTKYFEDMK SKCDPPKRAD TCGDNSNIEC KK ACANYTN WLNPKRIEWN GMSNYYNKIY RKSNKESEDG KDYSMIMAPT VIDYLNKRCH GEINGNYICC SCKNIGAYNT TSG TVNKKL QKKETECEEE KGPLDLMNEV LNKMDKKYSA HKMKCTEVYL EHVEEQLNEI DNAIKDYKLY PLDRCFDDQT KMKV CDLIA DAIGCKDKTK LDELDEWNDM DLRGTYNKHK GVLIPPRRRQ LCFSRIVRGP ANLRSLNEFK EEILKGAQSE GKFLG NYYK EHKDKEKALE AMKNSFYDYE DIIKGTDMLT NIEFKDIKIK LDRLLEKETN NTKKAEDWWK TNKKSIWNAM LCGYKK SGN KIIDPSWCTI PTTETPPQFL RWIKEWGTNV CIQKQEHKEY VKSKCSNVTN LGAQASESNN CTSEIKKYQE WSRKRSI QW ETISKRYKKY KRMDILKDVK EPDANTYLRE HCSKCPCGFN DMEEMNNNED NEKEAFKQIK EQVKIPAELE DVIYRIKH H EYDKGNDYIC NKYKNIHDRM KKNNGNFVTD NFVKKSWEIS NGVLIPPRRK NLFLYIDPSK ICEYKKDPKL FKDFIYWSA FTEVERLKKA YGGARAKVVH AMKYSFTDIG SIIKGDDMME KNSSDKIGKI LGDTDGQNEK RKKWWDMNKY HIWESMLCGY REAEGDTET NENCRFPDIE SVPQFLRWFQ EWSENFCDRR QKLYDKLNSE CISAECTNGS VDNSKCTHAC VNYKNYILTK K TEYEIQTN KYDNEFKNKN SNDKDAPDYL KEKCNDNKCE CLNKHIDDKN KTWKNPYETL EDTFKSKCDC PKPLPSPIKP DD LPPQADE PF UniProtKB: Erythrocyte membrane protein 1 |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 8.6 mg/mL |
|---|---|
| Buffer | pH: 7.5 / Details: 20mM Tris pH 7.5, 125mM KCl |
| Grid | Model: Quantifoil R1.2/1.3 / Material: COPPER / Mesh: 300 / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 30 sec. / Pretreatment - Atmosphere: AIR |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV / Details: Added 1mM FF8 as additive just before freezing. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: FEI FALCON III (4k x 4k) / Detector mode: INTEGRATING / Number grids imaged: 1 / Number real images: 8081 / Average electron dose: 60.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 100.0 µm / Illumination mode: OTHER / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller













 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)