[English] 日本語
 Yorodumi
Yorodumi- EMDB-0832: Structure of the human sterol O-acyltransferase 1 in resting state -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-0832 | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of the human sterol O-acyltransferase 1 in resting state | ||||||||||||||||||
 Map data Map data | postprocessed map from relion | ||||||||||||||||||
 Sample Sample |
| ||||||||||||||||||
 Keywords Keywords | SOAT / ACAT / MBOAT / MEMBRANE PROTEIN / TRANSFERASE | ||||||||||||||||||
| Function / homology |  Function and homology information Function and homology informationsterol O-acyltransferase / sterol O-acyltransferase activity / cholesterol O-acyltransferase activity / macrophage derived foam cell differentiation / cholesterol storage / positive regulation of amyloid precursor protein biosynthetic process / very-low-density lipoprotein particle assembly / fatty-acyl-CoA binding / low-density lipoprotein particle clearance / LDL clearance ...sterol O-acyltransferase / sterol O-acyltransferase activity / cholesterol O-acyltransferase activity / macrophage derived foam cell differentiation / cholesterol storage / positive regulation of amyloid precursor protein biosynthetic process / very-low-density lipoprotein particle assembly / fatty-acyl-CoA binding / low-density lipoprotein particle clearance / LDL clearance / cholesterol efflux / cholesterol binding / cholesterol metabolic process / cholesterol homeostasis / endoplasmic reticulum membrane / endoplasmic reticulum / identical protein binding / membrane Similarity search - Function | ||||||||||||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||||||||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.5 Å | ||||||||||||||||||
 Authors Authors | Chen L / Guan C | ||||||||||||||||||
| Funding support |  China, 5 items China, 5 items
| ||||||||||||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2020 Journal: Nat Commun / Year: 2020Title: Structural insights into the inhibition mechanism of human sterol O-acyltransferase 1 by a competitive inhibitor. Authors: Chengcheng Guan / Yange Niu / Si-Cong Chen / Yunlu Kang / Jing-Xiang Wu / Koji Nishi / Catherine C Y Chang / Ta-Yuan Chang / Tuoping Luo / Lei Chen /   Abstract: Sterol O-acyltransferase 1 (SOAT1) is an endoplasmic reticulum (ER) resident, multi-transmembrane enzyme that belongs to the membrane-bound O-acyltransferase (MBOAT) family. It catalyzes the ...Sterol O-acyltransferase 1 (SOAT1) is an endoplasmic reticulum (ER) resident, multi-transmembrane enzyme that belongs to the membrane-bound O-acyltransferase (MBOAT) family. It catalyzes the esterification of cholesterol to generate cholesteryl esters for cholesterol storage. SOAT1 is a target to treat several human diseases. However, its structure and mechanism remain elusive since its discovery. Here, we report the structure of human SOAT1 (hSOAT1) determined by cryo-EM. hSOAT1 is a tetramer consisted of a dimer of dimer. The structure of hSOAT1 dimer at 3.5 Å resolution reveals that a small molecule inhibitor CI-976 binds inside the catalytic chamber and blocks the accessibility of the active site residues H460, N421 and W420. Our results pave the way for future mechanistic study and rational drug design targeting hSOAT1 and other mammalian MBOAT family members. | ||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_0832.map.gz emd_0832.map.gz | 2.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-0832-v30.xml emd-0832-v30.xml emd-0832.xml emd-0832.xml | 10.9 KB 10.9 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_0832_fsc.xml emd_0832_fsc.xml | 7.2 KB | Display |  FSC data file FSC data file |
| Images |  emd_0832.png emd_0832.png | 265.3 KB | ||
| Filedesc metadata |  emd-0832.cif.gz emd-0832.cif.gz | 5.3 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-0832 http://ftp.pdbj.org/pub/emdb/structures/EMD-0832 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-0832 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-0832 | HTTPS FTP |
-Related structure data
| Related structure data |  6l48MC  0829C  0830C  0831C  6l47C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_0832.map.gz / Format: CCP4 / Size: 30.5 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_0832.map.gz / Format: CCP4 / Size: 30.5 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | postprocessed map from relion | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.045 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
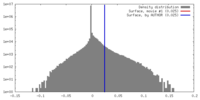
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : human sterol O-acyltransferase 1 dimer
| Entire | Name: human sterol O-acyltransferase 1 dimer |
|---|---|
| Components |
|
-Supramolecule #1: human sterol O-acyltransferase 1 dimer
| Supramolecule | Name: human sterol O-acyltransferase 1 dimer / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Sterol O-acyltransferase 1
| Macromolecule | Name: Sterol O-acyltransferase 1 / type: protein_or_peptide / ID: 1 / Number of copies: 2 / Enantiomer: LEVO / EC number: sterol O-acyltransferase |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 57.294777 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MKEVGSHFDD FVTNLIEKSA SLDNGGCALT TFSVLEGEKN NHRAKDLRAP PEQGKIFIAR RSLLDELLEV DHIRTIYHMF IALLILFIL STLVVDYIDE GRLVLEFSLL SYAFGKFPTV VWTWWIMFLS TFSVPYFLFQ HWATGYSKSS HPLIRSLFHG F LFMIFQIG ...String: MKEVGSHFDD FVTNLIEKSA SLDNGGCALT TFSVLEGEKN NHRAKDLRAP PEQGKIFIAR RSLLDELLEV DHIRTIYHMF IALLILFIL STLVVDYIDE GRLVLEFSLL SYAFGKFPTV VWTWWIMFLS TFSVPYFLFQ HWATGYSKSS HPLIRSLFHG F LFMIFQIG VLGFGPTYVV LAYTLPPASR FIIIFEQIRF VMKAHSFVRE NVPRVLNSAK EKSSTVPIPT VNQYLYFLFA PT LIYRDSY PRNPTVRWGY VAMKFAQVFG CFFYVYYIFE RLCAPLFRNI KQEPFSARVL VLCVFNSILP GVLILFLTFF AFL HCWLNA FAEMLRFGDR MFYKDWWNST SYSNYYRTWN VVVHDWLYYY AYKDFLWFFS KRFKSAAMLA VFAVSAVVHE YALA VCLSF FYPVLFVLFM FFGMAFNFIV NDSRKKPIWN VLMWTSLFLG NGVLLCFYSQ EWYARQHCPL KNPTFLDYVR PRSWT CRYV F UniProtKB: Sterol O-acyltransferase 1 |
-Macromolecule #2: CHOLESTEROL
| Macromolecule | Name: CHOLESTEROL / type: ligand / ID: 2 / Number of copies: 2 / Formula: CLR |
|---|---|
| Molecular weight | Theoretical: 386.654 Da |
| Chemical component information |  ChemComp-CLR: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 QUANTUM (4k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller














 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)