[English] 日本語
 Yorodumi
Yorodumi- EMDB-0830: Structure of the human sterol O-acyltransferase 1 tetramer in rho... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-0830 | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of the human sterol O-acyltransferase 1 tetramer in rhombic shape | ||||||||||||||||||
 Map data Map data | |||||||||||||||||||
 Sample Sample |
| ||||||||||||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||||||||||||||
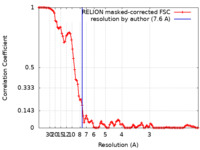
| Method | single particle reconstruction / cryo EM / Resolution: 7.6 Å | ||||||||||||||||||
 Authors Authors | Chen L / Guan C | ||||||||||||||||||
| Funding support |  China, 5 items China, 5 items
| ||||||||||||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2020 Journal: Nat Commun / Year: 2020Title: Structural insights into the inhibition mechanism of human sterol O-acyltransferase 1 by a competitive inhibitor. Authors: Chengcheng Guan / Yange Niu / Si-Cong Chen / Yunlu Kang / Jing-Xiang Wu / Koji Nishi / Catherine C Y Chang / Ta-Yuan Chang / Tuoping Luo / Lei Chen /   Abstract: Sterol O-acyltransferase 1 (SOAT1) is an endoplasmic reticulum (ER) resident, multi-transmembrane enzyme that belongs to the membrane-bound O-acyltransferase (MBOAT) family. It catalyzes the ...Sterol O-acyltransferase 1 (SOAT1) is an endoplasmic reticulum (ER) resident, multi-transmembrane enzyme that belongs to the membrane-bound O-acyltransferase (MBOAT) family. It catalyzes the esterification of cholesterol to generate cholesteryl esters for cholesterol storage. SOAT1 is a target to treat several human diseases. However, its structure and mechanism remain elusive since its discovery. Here, we report the structure of human SOAT1 (hSOAT1) determined by cryo-EM. hSOAT1 is a tetramer consisted of a dimer of dimer. The structure of hSOAT1 dimer at 3.5 Å resolution reveals that a small molecule inhibitor CI-976 binds inside the catalytic chamber and blocks the accessibility of the active site residues H460, N421 and W420. Our results pave the way for future mechanistic study and rational drug design targeting hSOAT1 and other mammalian MBOAT family members. | ||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_0830.map.gz emd_0830.map.gz | 20.5 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-0830-v30.xml emd-0830-v30.xml emd-0830.xml emd-0830.xml | 10.4 KB 10.4 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_0830_fsc.xml emd_0830_fsc.xml | 10.4 KB | Display |  FSC data file FSC data file |
| Images |  emd_0830.png emd_0830.png | 91.2 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-0830 http://ftp.pdbj.org/pub/emdb/structures/EMD-0830 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-0830 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-0830 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_0830.map.gz / Format: CCP4 / Size: 22.2 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_0830.map.gz / Format: CCP4 / Size: 22.2 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.045 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : human sterol O-acyltransferase 1 tetramer
| Entire | Name: human sterol O-acyltransferase 1 tetramer |
|---|---|
| Components |
|
-Supramolecule #1: human sterol O-acyltransferase 1 tetramer
| Supramolecule | Name: human sterol O-acyltransferase 1 tetramer / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Recombinant expression | Organism:  Homo sapiens (human) / Recombinant cell: HEK293T Homo sapiens (human) / Recombinant cell: HEK293T |
-Macromolecule #1: Structure of the human sterol O-acyltransferase 1
| Macromolecule | Name: Structure of the human sterol O-acyltransferase 1 / type: protein_or_peptide / ID: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MVGEEKMSLR NRLSKSRENP EEDEDQRNPA KESLETPSNG RIDIKQLIAK KIKLTAEAEE LKPFFMKEVG SHFDDFVTNL IEKSASLDN GGCALTTFSV LEGEKNNHRA KDLRAPPEQG KIFIARRSLL DELLEVDHIR TIYHMFIALL ILFILSTLVV D YIDEGRLV ...String: MVGEEKMSLR NRLSKSRENP EEDEDQRNPA KESLETPSNG RIDIKQLIAK KIKLTAEAEE LKPFFMKEVG SHFDDFVTNL IEKSASLDN GGCALTTFSV LEGEKNNHRA KDLRAPPEQG KIFIARRSLL DELLEVDHIR TIYHMFIALL ILFILSTLVV D YIDEGRLV LEFSLLSYAF GKFPTVVWTW WIMFLSTFSV PYFLFQHWAT GYSKSSHPLI RSLFHGFLFM IFQIGVLGFG PT YVVLAYT LPPASRFIII FEQIRFVMKA HSFVRENVPR VLNSAKEKSS TVPIPTVNQY LYFLFAPTLI YRDSYPRNPT VRW GYVAMK FAQVFGCFFY VYYIFERLCA PLFRNIKQEP FSARVLVLCV FNSILPGVLI LFLTFFAFLH CWLNAFAEML RFGD RMFYK DWWNSTSYSN YYRTWNVVVH DWLYYYAYKD FLWFFSKRFK SAAMLAVFAV SAVVHEYALA VCLSFFYPVL FVLFM FFGM AFNFIVNDSR KKPIWNVLMW TSLFLGNGVL LCFYSQEWYA RQHCPLKNPT FLDYVRPRSW TCRYVF |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 QUANTUM (4k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
-Atomic model buiding 1
| Refinement | Protocol: AB INITIO MODEL |
|---|
 Movie
Movie Controller
Controller














 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)