+Search query
-Structure paper
| Title | Mechanistic insights into single-stranded DNA degradation by lysosomal exonucleases PLD3 and PLD4 from structural snapshots. |
|---|---|
| Journal, issue, pages | Nat Commun, Vol. 16, Issue 1, Page 11431, Year 2025 |
| Publish date | Dec 11, 2025 |
 Authors Authors | Yoshinori Hirano / Wakiko Ezaki / Ryota Sato / Umeharu Ohto / Kensuke Miyake / Toshiyuki Shimizu /  |
| PubMed Abstract | Lysosomal exonuclease phospholipase D (PLD) family PLD3 and PLD4 degrade single-stranded RNA or DNA and regulate TLR7 or TLR9 responses. Polymorphisms of these enzymes are associated with human ...Lysosomal exonuclease phospholipase D (PLD) family PLD3 and PLD4 degrade single-stranded RNA or DNA and regulate TLR7 or TLR9 responses. Polymorphisms of these enzymes are associated with human diseases: PLD4 is associated with inflammatory diseases, and PLD3 is associated with neurodegenerative diseases. Here, we determine the structures of substrate-bound PLD3 and PLD4 by cryo-electron microscopy. Our structures reveal that PLD3 rebuilds a substrate-binding pocket, depending on the substrate, mainly via motion of the Phe335-containing loop. Furthermore, we captured the structure in a metastable state that appears during substrate rearrangement following product release. Together, our findings identify the residues that underlie the distinct activities of PLD3 and PLD4. This study provides a mechanistic basis for the exonuclease activity of PLD3 and PLD4 in single-stranded DNA degradation. |
 External links External links |  Nat Commun / Nat Commun /  PubMed:41381514 / PubMed:41381514 /  PubMed Central PubMed Central |
| Methods | EM (single particle) |
| Resolution | 2.85 - 3.31 Å |
| Structure data | EMDB-64921, PDB-9vbg: EMDB-64922, PDB-9vbh: EMDB-64923, PDB-9vbi: EMDB-64924, PDB-9vbj: EMDB-64925, PDB-9vbk: |
| Chemicals |  ChemComp-NAG: 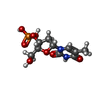 ChemComp-T3P: |
| Source |
|
 Keywords Keywords | IMMUNE SYSTEM / Exonuclease |
 Movie
Movie Controller
Controller Structure viewers
Structure viewers About Yorodumi Papers
About Yorodumi Papers













 homo sapiens (human)
homo sapiens (human)