+Search query
-Structure paper
| Title | Phosphoantigen-induced inside-out stabilization of butyrophilin receptor complexes drives dimerization-dependent γδ TCR activation. |
|---|---|
| Journal, issue, pages | Immunity, Vol. 58, Issue 7, Page 1646-11659.e5, Year 2025 |
| Publish date | Jul 8, 2025 |
 Authors Authors | Yuwei Zhu / Wenbo Gao / Jianlin Zheng / Ye Bai / Xinyu Tian / Tengjin Huang / Zebin Lu / De Dong / Anqi Zhang / Changyou Guo / Zhiwei Huang /  |
| PubMed Abstract | Phosphoantigens (pAgs), produced by infected or cancer cells, trigger the assembly of a membrane receptor complex comprising butyrophilin (BTN) members BTN3A1 and BTN2A1, leading to the activation of ...Phosphoantigens (pAgs), produced by infected or cancer cells, trigger the assembly of a membrane receptor complex comprising butyrophilin (BTN) members BTN3A1 and BTN2A1, leading to the activation of γδ T cells. BTN3A2 or BTN3A3 forms heteromers with BTN3A1, exhibiting higher γδ T cell receptor (TCR)-stimulating activity than BTN3A1 homomers. Cryoelectron microscopy (cryo-EM) structure reveals a pAg-induced BTN2A1-BTN3A1 heterotetramer with a 2:2 stoichiometry, stabilized by interactions between the intracellular B30.2 domains and the extracellular immunoglobulin V (IgV) domains. BTN3A2 or BTN3A3 heterodimerizes with BTN3A1, forming a pAg-induced tetrameric complex with BTN2A1. However, BTN3A1 heterodimers are more stable than BTN3A1 homodimers in this interaction. Cryo-EM reveals that BTN2A1-BTN3A1-BTN3A2 binds two γδ TCR ectodomains, with one being sandwiched between the IgV domains of BTN2A1 and BTN3A2, while the other interacts with the free BTN2A1 IgV in the complex, as evidenced by functional data. Together, our findings uncover the mechanism of ligand-induced inside-out stabilization of BTN receptor complexes for dimeric activation of γδ TCR. |
 External links External links |  Immunity / Immunity /  PubMed:40334665 PubMed:40334665 |
| Methods | EM (single particle) |
| Resolution | 2.9 - 6.95 Å |
| Structure data | EMDB-39874, PDB-8za6: EMDB-39875, PDB-8za9: EMDB-39876, PDB-8zaa: EMDB-39877, PDB-8zab: EMDB-39950, PDB-8zd4:  EMDB-60027: Cryo-EM structure of the Vg9Vd2 TCR-CD3 EMDB-60577, PDB-8zyr: EMDB-60591, PDB-9ii6: EMDB-60596, PDB-9iik: EMDB-61143, PDB-9j5j: EMDB-61146, PDB-9j5m: |
| Chemicals |  ChemComp-CLR: 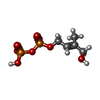 ChemComp-H6P: 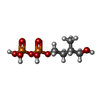 ChemComp-EIP: |
| Source |
|
 Keywords Keywords | IMMUNE SYSTEM / complex / immune / protein |
 Movie
Movie Controller
Controller Structure viewers
Structure viewers About Yorodumi Papers
About Yorodumi Papers























 homo sapiens (human)
homo sapiens (human)