+Search query
-Structure paper
| Title | Differential activation mechanisms of lipid GPCRs by lysophosphatidic acid and sphingosine 1-phosphate. |
|---|---|
| Journal, issue, pages | Nat Commun, Vol. 13, Issue 1, Page 731, Year 2022 |
| Publish date | Feb 8, 2022 |
 Authors Authors | Shian Liu / Navid Paknejad / Lan Zhu / Yasuyuki Kihara / Manisha Ray / Jerold Chun / Wei Liu / Richard K Hite / Xin-Yun Huang /  |
| PubMed Abstract | Lysophospholipids are bioactive lipids and can signal through G-protein-coupled receptors (GPCRs). The best studied lysophospholipids are lysophosphatidic acid (LPA) and sphingosine 1-phosphate (S1P). ...Lysophospholipids are bioactive lipids and can signal through G-protein-coupled receptors (GPCRs). The best studied lysophospholipids are lysophosphatidic acid (LPA) and sphingosine 1-phosphate (S1P). The mechanisms of lysophospholipid recognition by an active GPCR, and the activations of lysophospholipid GPCR-G-protein complexes remain unclear. Here we report single-particle cryo-EM structures of human S1P receptor 1 (S1P) and heterotrimeric G complexes formed with bound S1P or the multiple sclerosis (MS) treatment drug Siponimod, as well as human LPA receptor 1 (LPA) and G complexes in the presence of LPA. Our structural and functional data provide insights into how LPA and S1P adopt different conformations to interact with their cognate GPCRs, the selectivity of the homologous lipid GPCRs for S1P versus LPA, and the different activation mechanisms of these GPCRs by LPA and S1P. Our studies also reveal specific optimization strategies to improve the MS-treating S1P-targeting drugs. |
 External links External links |  Nat Commun / Nat Commun /  PubMed:35136060 / PubMed:35136060 /  PubMed Central PubMed Central |
| Methods | EM (single particle) |
| Resolution | 2.6 - 3.11 Å |
| Structure data | EMDB-25819, PDB-7td0: EMDB-25820, PDB-7td1: EMDB-25821, PDB-7td2: EMDB-25822, PDB-7td3: EMDB-25823, PDB-7td4: |
| Chemicals | 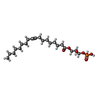 ChemComp-NKP: 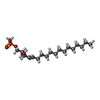 ChemComp-S1P:  ChemComp-NAG:  ChemComp-J8C: |
| Source |
|
 Keywords Keywords | MEMBRANE PROTEIN / GPCR / complex / lipid |
 Movie
Movie Controller
Controller Structure viewers
Structure viewers About Yorodumi Papers
About Yorodumi Papers














 homo sapiens (human)
homo sapiens (human)