+Search query
-Structure paper
| Title | Tubulin code eraser CCP5 binds branch glutamates by substrate deformation. |
|---|---|
| Journal, issue, pages | Nature, Vol. 631, Issue 8022, Page 905-912, Year 2024 |
| Publish date | Jul 17, 2024 |
 Authors Authors | Jiayi Chen / Elena A Zehr / James M Gruschus / Agnieszka Szyk / Yanjie Liu / Martin E Tanner / Nico Tjandra / Antonina Roll-Mecak /   |
| PubMed Abstract | Microtubule function is modulated by the tubulin code, diverse posttranslational modifications that are altered dynamically by writer and eraser enzymes. Glutamylation-the addition of branched ...Microtubule function is modulated by the tubulin code, diverse posttranslational modifications that are altered dynamically by writer and eraser enzymes. Glutamylation-the addition of branched (isopeptide-linked) glutamate chains-is the most evolutionarily widespread tubulin modification. It is introduced by tubulin tyrosine ligase-like enzymes and erased by carboxypeptidases of the cytosolic carboxypeptidase (CCP) family. Glutamylation homeostasis, achieved through the balance of writers and erasers, is critical for normal cell function, and mutations in CCPs lead to human disease. Here we report cryo-electron microscopy structures of the glutamylation eraser CCP5 in complex with the microtubule, and X-ray structures in complex with transition-state analogues. Combined with NMR analysis, these analyses show that CCP5 deforms the tubulin main chain into a unique turn that enables lock-and-key recognition of the branch glutamate in a cationic pocket that is unique to CCP family proteins. CCP5 binding of the sequences flanking the branch point primarily through peptide backbone atoms enables processing of diverse tubulin isotypes and non-tubulin substrates. Unexpectedly, CCP5 exhibits inefficient processing of an abundant β-tubulin isotype in the brain. This work provides an atomistic view into glutamate branch recognition and resolution, and sheds light on homeostasis of the tubulin glutamylation syntax. |
 External links External links |  Nature / Nature /  PubMed:39020174 PubMed:39020174 |
| Methods | EM (single particle) / X-ray diffraction |
| Resolution | 1.8 - 3.6 Å |
| Structure data | EMDB-42950, PDB-8v3q: EMDB-42951, PDB-8v3r: EMDB-42952, PDB-8v3s: EMDB-42971, PDB-8v4k: EMDB-42972, PDB-8v4l: EMDB-42973, PDB-8v4m:  PDB-8v3m:  PDB-8v3n:  PDB-8v3o:  PDB-8v3p: |
| Chemicals | 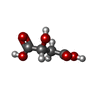 ChemComp-MLT:  ChemComp-IMD:  ChemComp-ZN:  ChemComp-HOH:  PDB-1aag:  ChemComp-K:  ChemComp-GLU:  ChemComp-MG:  ChemComp-GTP:  ChemComp-G2P: |
| Source |
|
 Keywords Keywords | HYDROLASE / carboxypeptidase deglutamylation branch glutamate removal microtubule / HYDROLASE/INHIBITOR / HYDROLASE-INHIBITOR complex / carboxypeptidase / deglutamylation / branch glutamate removal / microtubule / HYDROLASE/SUBSTRATE / HYDROLASE-SUBSTRATE complex / STRUCTURAL PROTEIN / HYDROLASE-SUBSTRATE / STRUCTURAL PROTEIN complex |
 Movie
Movie Controller
Controller Structure viewers
Structure viewers About Yorodumi Papers
About Yorodumi Papers



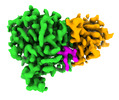

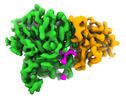

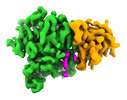




 homo sapiens (human)
homo sapiens (human)
