+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-9664 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Tra1 subunit from Saccharomyces cerevisiae SAGA complex | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Epigenetics / Acetyltransferase / Transcription / Co-activator | |||||||||
| Function / homology | :  Function and homology information Function and homology information | |||||||||
| Biological species |  | |||||||||
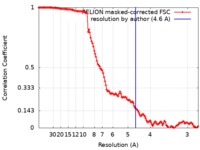
| Method | single particle reconstruction / cryo EM / Resolution: 4.6 Å | |||||||||
 Authors Authors | Zheng XD / Liu GC | |||||||||
 Citation Citation |  Journal: Cell Discov / Year: 2019 Journal: Cell Discov / Year: 2019Title: Architecture of SAGA complex. Authors: Gaochao Liu / Xiangdong Zheng / Haipeng Guan / Yong Cao / Hongyuan Qu / Junqing Kang / Xiangle Ren / Jianlin Lei / Meng-Qiu Dong / Xueming Li / Haitao Li /  | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_9664.map.gz emd_9664.map.gz | 7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-9664-v30.xml emd-9664-v30.xml emd-9664.xml emd-9664.xml | 25 KB 25 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_9664_fsc.xml emd_9664_fsc.xml | 12.8 KB | Display |  FSC data file FSC data file |
| Images |  emd_9664.png emd_9664.png | 47.9 KB | ||
| Masks |  emd_9664_msk_1.map emd_9664_msk_1.map | 178 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-9664.cif.gz emd-9664.cif.gz | 8.4 KB | ||
| Others |  emd_9664_additional.map.gz emd_9664_additional.map.gz emd_9664_half_map_1.map.gz emd_9664_half_map_1.map.gz emd_9664_half_map_2.map.gz emd_9664_half_map_2.map.gz | 7 MB 140.7 MB 140.7 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-9664 http://ftp.pdbj.org/pub/emdb/structures/EMD-9664 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-9664 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-9664 | HTTPS FTP |
-Validation report
| Summary document |  emd_9664_validation.pdf.gz emd_9664_validation.pdf.gz | 579.9 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_9664_full_validation.pdf.gz emd_9664_full_validation.pdf.gz | 579.5 KB | Display | |
| Data in XML |  emd_9664_validation.xml.gz emd_9664_validation.xml.gz | 19.9 KB | Display | |
| Data in CIF |  emd_9664_validation.cif.gz emd_9664_validation.cif.gz | 26 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-9664 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-9664 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-9664 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-9664 | HTTPS FTP |
-Related structure data
| Related structure data |  6ig9MC  9663C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_9664.map.gz / Format: CCP4 / Size: 178 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_9664.map.gz / Format: CCP4 / Size: 178 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.401 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Mask #1
| File |  emd_9664_msk_1.map emd_9664_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: #1
| File | emd_9664_additional.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_9664_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_9664_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Tra1 / ScTra1
| Entire | Name: Tra1 / ScTra1 |
|---|---|
| Components |
|
-Supramolecule #1: Tra1 / ScTra1
| Supramolecule | Name: Tra1 / ScTra1 / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all Details: One of the major part from SAGA (Spt-Ada-Gcn5-Acetyltransferase) complex |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 1.8 MDa |
-Macromolecule #1: Transcription-associated protein 1
| Macromolecule | Name: Transcription-associated protein 1 / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 433.677281 KDa |
| Sequence | String: MSLTEQIEQF ASRFRDDDAT LQSRYSTLSE LYDIMELLNS PEDYHFFLQA VIPLLLNQLK EVPISYDAHS PEQKLRNSML DIFNRCLMN QTFQPYAMEV LEFLLSVLPK ENEENGILCM KVLTTLFKSF KSILQDKLDS FIRIIIQIYK NTPNLINQTF Y EAGKAEQG ...String: MSLTEQIEQF ASRFRDDDAT LQSRYSTLSE LYDIMELLNS PEDYHFFLQA VIPLLLNQLK EVPISYDAHS PEQKLRNSML DIFNRCLMN QTFQPYAMEV LEFLLSVLPK ENEENGILCM KVLTTLFKSF KSILQDKLDS FIRIIIQIYK NTPNLINQTF Y EAGKAEQG DLDSPKEPQA DELLDEFSKN DEEKDFPSKQ SSTEPRFENS TSSNGLRSSM FSFKILSECP ITMVTLYSSY KQ LTSTSLP EFTPLIMNLL NIQIKQQQEA REQAESRGEH FTSISTEIIN RPAYCDFILA QIKATSFLAY VFIRGYAPEF LQD YVNFVP DLIIRLLQDC PSELSSARKE LLHATRHILS TNYKKLFLPK LDYLFDERIL IGNGFTMHET LRPLAYSTVA DFIH NIRSE LQLSEIEKTI KIYTGYLLDE SLALTVQIMS AKLLLNLVER ILKLGKENPQ EAPRAKKLLM IIIDSYMNRF KTLNR QYDT IMKYYGRYET HKKEKAEKLK NSIQDNDKES EEFMRKVLEP SDDDHLMPQP KKEDINDSPD VEMTESDKVV KNDVEM FDI KNYAPILLLP TPTNDPIKDA FYLYRTLMSF LKTIIHDLKV FNPPPNEYTV ANPKLWASVS RVFSYEEVIV FKDLFHE CI IGLKFFKDHN EKLSPETTKK HFDISMPSLP VSATKDAREL MDYLAFMFMQ MDNATFNEII EQELPFVYER MLEDSGLL H VAQSFLTSEI TSPNFAGILL RFLKGKLKDL GNVDFNTSNV LIRLFKLSFM SVNLFPNINE VVLLPHLNDL ILNSLKYST TAEEPLVYFY LIRTLFRSIG GGRFENLYRS IKPILQVLLQ SLNQMILTAR LPHERELYVE LCITVPVRLS VLAPYLPFLM KPLVFALQQ YPDLVSQGLR TLELCIDNLT AEYFDPIIEP VIDDVSKALF NLLQPQPFNH AISHNVVRIL GKLGGRNRQF L KPPTDLTE KTELDIDAIA DFKINGMPED VPLSVTPGIQ SALNILQSYK SDIHYRKSAY KYLTCVLLLM TKSSAEFPTN YT ELLKTAV NSIKLERIGI EKNFDLEPTV NKRDYSNQEN LFLRLLESVF YATSIKELKD DAMDLLNNLL DHFCLLQVNT TLL NKRNYN GTFNIDLKNP NFMLDSSLIL DAIPFALSYY IPEVREVGVL AYKRIYEKSC LIYGEELALS HSFIPELAKQ FIHL CYDET YYNKRGGVLG IKVLIDNVKS SSVFLKKYQY NLANGLLFVL KDTQSEAPSA ITDSAEKLLI DLLSITFADV KEEDL GNKV LENTLTDIVC ELSNANPKVR NACQKSLHTI SNLTGIPIVK LMDHSKQFLL SPIFAKPLRA LPFTMQIGNV DAITFC LSL PNTFLTFNEE LFRLLQESIV LADAEDESLS TNIQKTTEYS TSEQLVQLRI ACIKLLAIAL KNEEFATAQQ GNIRIRI LA VFFKTMLKTS PEIINTTYEA LKGSLAENSK LPKELLQNGL KPLLMNLSDH QKLTVPGLDA LSKLLELLIA YFKVEIGR K LLDHLTAWCR VEVLDTLFGQ DLAEQMPTKI IVSIINIFHL LPPQADMFLN DLLLKVMLLE RKLRLQLDSP FRTPLARYL NRFHNPVTEY FKKNMTLRQL VLFMCNIVQR PEAKELAEDF EKELDNFYDF YISNIPKNQV RVVSFFTNMV DLFNTMVITN GDEWLKKKG NMILKLKDML NLTLKTIKEN SFYIDHLQLN QSIAKFQALY LRFTELSERD QNPLLLDFID FSFSNGIKAS Y SLKKFIFH NIIASSNKEK QNNFINDATL FVLSDKCLDA RIFVLKNVIN STLIYEVATS GSLKSYLVED KKPKWLELLH NK IWKNSNA ILAYDVLDHH DLFRFELLQL SAIFIKADPE IIAEIKKDII KFCWNFIKLE DTLIKQSAYL VTSYFISKFD FPI KVVTQV FVALLRSSHV EARYLVKQSL DVLTPVLHER MNAAGTPDTW INWVKRVMVE NSSSQNNILY QFLISHPDLF FNSR DLFIS NIIHHMNKIT FMSNSNSDSH TLAIDLASLI LYWENKTLEI TNVNNTKTDS DGDVVMSDSK SDINPVEADT TAIIV DANN NSPISLHLRE ACTAFLIRYV CASNHRAIET ELGLRAINIL SELISDKHWT NVNVKLVYFE KFLIFQDLDS ENILYY CMN ALDVLYVFFK NKTKEWIMEN LPTIQNLLEK CIKSDHHDVQ EALQKVLQVI MKAIKAQGVS VIIEEESPGK TFIQMLT SV ITQDLQETSS VTAGVTLAWV LFMNFPDNIV PLLTPLMKTF SKLCKDHLSI SQPKDAMALE EARITTKLLE KVLYILSL K VSLLGDSRRP FLSTVALLID HSMDQNFLRK IVNMSRSWIF NTEIFPTVKE KAAILTKMLA FEIRGEPSLS KLFYEIVLK LFDQEHFNNT EITVRMEQPF LVGTRVEDIG IRKRFMTILD NSLERDIKER LYYVIRDQNW EFIADYPWLN QALQLLYGSF NREKELSLK NIYCLSPPSI LQEYLPENAE MVTEVNDLEL SNFVKGHIAS MQGLCRIISS DFIDSLIEIF YQDPKAIHRA W VTLFPQVY KSIPKNEKYG FVRSIITLLS KPYHTRQISS RTNVINMLLD SISKIESLEL PPHLVKYLAI SYNAWYQSIN IL ESIQSNT SIDNTKIIEA NEDALLELYV NLQEEDMFYG LWRRRAKYTE TNIGLSYEQI GLWDKAQQLY EVAQVKARSG ALP YSQSEY ALWEDNWIQC AEKLQHWDVL TELAKHEGFT DLLLECGWRV ADWNSDRDAL EQSVKSVMDV PTPRRQMFKT FLAL QNFAE SRKGDQEVRK LCDEGIQLSL IKWVSLPIRY TPAHKWLLHG FQQYMEFLEA TQIYANLHTT TVQNLDSKAQ EIKRI LQAW RDRLPNTWDD VNMWNDLVTW RQHAFQVINN AYLPLIPALQ QSNSNSNINT HAYRGYHEIA WVINRFAHVA RKHNMP DVC ISQLARIYTL PNIEIQEAFL KLREQAKCHY QNMNELTTGL DVISNTNLVY FGTVQKAEFF TLKGMFLSKL RAYEEAN QA FATAVQIDLN LAKAWAQWGF FNDRRLSEEP NNISFASNAI SCYLQAAGLY KNSKIRELLC RILWLISIDD ASGMLTNA F DSFRGEIPVW YWITFIPQLL TSLSHKEANM VRHILIRIAK SYPQALHFQL RTTKEDFAVI QRQTMAVMGD KPDTNDRNG RRQPWEYLQE LNNILKTAYP LLALSLESLV AQINDRFKST TDEDLFRLIN VLLIDGTLNY NRLPFPRKNP KLPENTEKNL VKFSTTLLA PYIRPKFNAD FIDNKPDYET YIKRLRYWRR RLENKLDRAS KKENLEVLCP HLSNFHHQKF EDIEIPGQYL L NKDNNVHF IKIARFLPTV DFVRGTHSSY RRLMIRGHDG SVHSFAVQYP AVRHSRREER MFQLYRLFNK SLSKNVETRR RS IQFNLPI AIPLSPQVRI MNDSVSFTTL HEIHNEFCKK KGFDPDDIQD FMADKLNAAH DDALPAPDMT ILKVEIFNSI QTM FVPSNV LKDHFTSLFT QFEDFWLFRK QFASQYSSFV FMSYMMMINN RTPHKIHVDK TSGNVFTLEM LPSRFPYERV KPLL KNHDL SLPPDSPIFH NNEPVPFRLT PNIQSLIGDS ALEGIFAVNL FTISRALIEP DNELNTYLAL FIRDEIISWF SNLHR PIIE NPQLREMVQT NVDLIIRKVA QLGHLNSTPT VTTQFILDCI GSAVSPRNLA RTDVNFMPWF UniProtKB: UNIPROTKB: P38811 |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.2 mg/mL | ||||||
|---|---|---|---|---|---|---|---|
| Buffer | pH: 8.5 / Component:
| ||||||
| Grid | Model: Quantifoil R2/2 / Material: COPPER / Mesh: 400 / Support film - Material: CARBON / Support film - topology: HOLEY / Support film - Film thickness: 5 / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 60 sec. / Pretreatment - Atmosphere: AIR / Pretreatment - Pressure: 101.325 kPa Details: The grid was coated with a home-made thin continuous carbon film. | ||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 95 % / Chamber temperature: 281 K / Instrument: FEI VITROBOT MARK IV Details: Blot for 4 seconds and wait for 30 seconds before plunging. | ||||||
| Details | We extracted it from the overall SAGA complex structure. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Temperature | Min: 70.0 K / Max: 70.0 K |
| Specialist optics | Spherical aberration corrector: With a Cs-corrector |
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: SUPER-RESOLUTION / Digitization - Dimensions - Width: 3838 pixel / Digitization - Dimensions - Height: 3710 pixel / Digitization - Frames/image: 3-30 / Number real images: 8526 / Average exposure time: 0.25 sec. / Average electron dose: 5.6 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 70.0 µm / Calibrated defocus max: 3.0 µm / Calibrated defocus min: 1.2 µm / Calibrated magnification: 81000 / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 0.1 mm / Nominal defocus max: 3.0 µm / Nominal defocus min: 1.2 µm / Nominal magnification: 81000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller












 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)