[English] 日本語
 Yorodumi
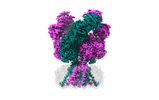
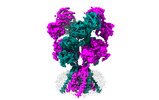
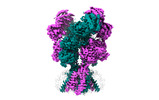
Yorodumi- EMDB-44586: Rat GluN1-GluN2B NMDA receptor channel in complex with glycine, g... -
+ Open data
Open data
- Basic information
Basic information
| Entry | 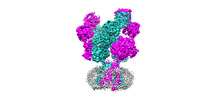 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Rat GluN1-GluN2B NMDA receptor channel in complex with glycine, glutamate, and EU-1622-A, in open-channel conformation, C1 symmetry | |||||||||
 Map data Map data | B-factor sharpened map | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Ionotropic glutamate receptor / synaptic membrane protein / MEMBRANE PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationorganelle / pons maturation / regulation of cell communication / positive regulation of Schwann cell migration / EPHB-mediated forward signaling / Assembly and cell surface presentation of NMDA receptors / olfactory learning / conditioned taste aversion / dendritic branch / regulation of respiratory gaseous exchange ...organelle / pons maturation / regulation of cell communication / positive regulation of Schwann cell migration / EPHB-mediated forward signaling / Assembly and cell surface presentation of NMDA receptors / olfactory learning / conditioned taste aversion / dendritic branch / regulation of respiratory gaseous exchange / protein localization to postsynaptic membrane / propylene metabolic process / response to glycine / voltage-gated monoatomic cation channel activity / regulation of monoatomic cation transmembrane transport / NMDA glutamate receptor activity / response to morphine / Synaptic adhesion-like molecules / NMDA selective glutamate receptor complex / RAF/MAP kinase cascade / parallel fiber to Purkinje cell synapse / calcium ion transmembrane import into cytosol / glutamate binding / neuromuscular process / positive regulation of reactive oxygen species biosynthetic process / protein heterotetramerization / regulation of synapse assembly / glycine binding / positive regulation of calcium ion transport into cytosol / regulation of axonogenesis / regulation of dendrite morphogenesis / male mating behavior / suckling behavior / startle response / response to amine / regulation of neuronal synaptic plasticity / monoatomic cation transmembrane transport / monoatomic cation transport / associative learning / positive regulation of excitatory postsynaptic potential / social behavior / ligand-gated monoatomic ion channel activity / excitatory synapse / cellular response to glycine / positive regulation of dendritic spine maintenance / Unblocking of NMDA receptors, glutamate binding and activation / phosphatase binding / cellular response to manganese ion / glutamate receptor binding / long-term memory / positive regulation of synaptic transmission, glutamatergic / prepulse inhibition / monoatomic cation channel activity / calcium ion homeostasis / regulation of neuron apoptotic process / synaptic cleft / glutamate-gated receptor activity / glutamate-gated calcium ion channel activity / presynaptic active zone membrane / response to fungicide / sensory perception of pain / dendrite membrane / ligand-gated monoatomic ion channel activity involved in regulation of presynaptic membrane potential / response to amphetamine / ionotropic glutamate receptor signaling pathway / hippocampal mossy fiber to CA3 synapse / adult locomotory behavior / regulation of membrane potential / excitatory postsynaptic potential / learning / transmitter-gated monoatomic ion channel activity involved in regulation of postsynaptic membrane potential / synaptic membrane / synaptic transmission, glutamatergic / postsynaptic density membrane / regulation of long-term neuronal synaptic plasticity / response to calcium ion / visual learning / calcium channel activity / regulation of synaptic plasticity / terminal bouton / response to organic cyclic compound / memory / cerebral cortex development / synaptic vesicle membrane / intracellular calcium ion homeostasis / neuron cellular homeostasis / rhythmic process / calcium ion transport / synaptic vesicle / signaling receptor activity / presynaptic membrane / amyloid-beta binding / protein-containing complex assembly / chemical synaptic transmission / postsynaptic membrane / response to ethanol / negative regulation of neuron apoptotic process / transcription by RNA polymerase II / learning or memory / dendritic spine Similarity search - Function | |||||||||
| Biological species |  | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.81 Å | |||||||||
 Authors Authors | Chou T-H / Furukawa H | |||||||||
| Funding support |  United States, 2 items United States, 2 items
| |||||||||
 Citation Citation |  Journal: Nature / Year: 2024 Journal: Nature / Year: 2024Title: Molecular mechanism of ligand gating and opening of NMDA receptor. Authors: Tsung-Han Chou / Max Epstein / Russell G Fritzemeier / Nicholas S Akins / Srinu Paladugu / Elijah Z Ullman / Dennis C Liotta / Stephen F Traynelis / Hiro Furukawa /  Abstract: Glutamate transmission and activation of ionotropic glutamate receptors are the fundamental means by which neurons control their excitability and neuroplasticity. The N-methyl-D-aspartate receptor ...Glutamate transmission and activation of ionotropic glutamate receptors are the fundamental means by which neurons control their excitability and neuroplasticity. The N-methyl-D-aspartate receptor (NMDAR) is unique among all ligand-gated channels, requiring two ligands-glutamate and glycine-for activation. These receptors function as heterotetrameric ion channels, with the channel opening dependent on the simultaneous binding of glycine and glutamate to the extracellular ligand-binding domains (LBDs) of the GluN1 and GluN2 subunits, respectively. The exact molecular mechanism for channel gating by the two ligands has been unclear, particularly without structures representing the open channel and apo states. Here we show that the channel gate opening requires tension in the linker connecting the LBD and transmembrane domain (TMD) and rotation of the extracellular domain relative to the TMD. Using electron cryomicroscopy, we captured the structure of the GluN1-GluN2B (GluN1-2B) NMDAR in its open state bound to a positive allosteric modulator. This process rotates and bends the pore-forming helices in GluN1 and GluN2B, altering the symmetry of the TMD channel from pseudofourfold to twofold. Structures of GluN1-2B NMDAR in apo and single-liganded states showed that binding of either glycine or glutamate alone leads to distinct GluN1-2B dimer arrangements but insufficient tension in the LBD-TMD linker for channel opening. This mechanistic framework identifies a key determinant for channel gating and a potential pharmacological strategy for modulating NMDAR activity. | |||||||||
| History |
|
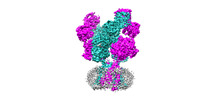
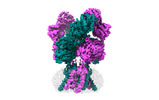
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_44586.map.gz emd_44586.map.gz | 230.3 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-44586-v30.xml emd-44586-v30.xml emd-44586.xml emd-44586.xml | 21.3 KB 21.3 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_44586.png emd_44586.png | 35.6 KB | ||
| Filedesc metadata |  emd-44586.cif.gz emd-44586.cif.gz | 7.1 KB | ||
| Others |  emd_44586_additional_1.map.gz emd_44586_additional_1.map.gz emd_44586_half_map_1.map.gz emd_44586_half_map_1.map.gz emd_44586_half_map_2.map.gz emd_44586_half_map_2.map.gz | 230 MB 226.4 MB 226.4 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-44586 http://ftp.pdbj.org/pub/emdb/structures/EMD-44586 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-44586 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-44586 | HTTPS FTP |
-Related structure data
| Related structure data |  9bibMC  9areC  9arfC  9argC  9arhC  9ariC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_44586.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_44586.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | B-factor sharpened map | ||||||||||||||||||||
| Voxel size | X=Y=Z: 0.861 Å | ||||||||||||||||||||
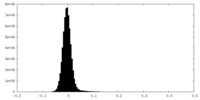
| Density |
| ||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Additional map: Local refined map focused on TMD
| File | emd_44586_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Local refined map focused on TMD | ||||||||||||
| Projections & Slices |
| ||||||||||||
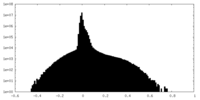
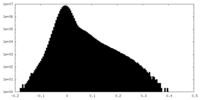
| Density Histograms |
-Half map: Half map B
| File | emd_44586_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map B | ||||||||||||
| Projections & Slices |
| ||||||||||||
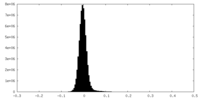
| Density Histograms |
-Half map: Half map A
| File | emd_44586_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map A | ||||||||||||
| Projections & Slices |
| ||||||||||||
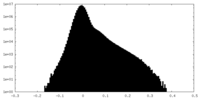
| Density Histograms |
- Sample components
Sample components
-Entire : Di-heterotetrameric GluN1-GluN2B NMDA receptors
| Entire | Name: Di-heterotetrameric GluN1-GluN2B NMDA receptors |
|---|---|
| Components |
|
-Supramolecule #1: Di-heterotetrameric GluN1-GluN2B NMDA receptors
| Supramolecule | Name: Di-heterotetrameric GluN1-GluN2B NMDA receptors / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#2 |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 400 KDa |
-Macromolecule #1: Glutamate receptor ionotropic, NMDA 1
| Macromolecule | Name: Glutamate receptor ionotropic, NMDA 1 / type: protein_or_peptide / ID: 1 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 95.225883 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MSTMHLLTFA LLFSCSFARA ASDPKIVNIG AVLSTRKHEQ MFREAVNQAN KRHGSWKIQL QATSVTHKPN AIQMALSVCE DLISSQVYA ILVSHPPTPN DHFTPTPVSY TAGFYRIPVL GLTTRMSIYS DKSIHLSFLR TVPPYSHQSS VWFEMMRVYN W NHIILLVS ...String: MSTMHLLTFA LLFSCSFARA ASDPKIVNIG AVLSTRKHEQ MFREAVNQAN KRHGSWKIQL QATSVTHKPN AIQMALSVCE DLISSQVYA ILVSHPPTPN DHFTPTPVSY TAGFYRIPVL GLTTRMSIYS DKSIHLSFLR TVPPYSHQSS VWFEMMRVYN W NHIILLVS DDHEGRAAQK RLETLLEERE SKAEKVLQFD PGTKNVTALL MEARELEARV IILSASEDDA ATVYRAAAML DM TGSGYVW LVGEREISGN ALRYAPDGII GLQLINGKNE SAHISDAVGV VAQAVHELLE KENITDPPRG CVGNTNIWKT GPL FKRVLM SSKYADGVTG RVEFNEDGDR KFAQYSIMNL QNRKLVQVGI YNGTHVIPND RKIIWPGGET EKPRGYQMST RLKI VTIHQ EPFVYVKPTM SDGTCKEEFT VNGDPVKKVI CTGPNDTSPG SPRHTVPQCC YGFCIDLLIK LARTMQFTYE VHLVA DGKF GTQERVQNSN KKEWNGMMGE LLSGQADMIV APLTINNERA QYIEFSKPFK YQGLTILVKK EIPRSTLDSF MQPFQS TLW LLVGLSVHVV AVMLYLLDRF SPFGRFKVNS EEEEEDALTL SSAMWFSWGV LLNSGIGEGA PRSFSARILG MVWAGFA MI IVASYTANLA AFLVLDRPEE RITGINDPRL RNPSDKFIYA TVKQSSVDIY FRRQVELSTM YRHMEKHNYE SAAEAIQA V RDNKLHAFIW DSAVLEFEAS QKCDLVTTGE LFFRSGFGIG MRKDSPWKQQ VSLSILKSHE NGFMEDLDKT WVRYQECDS RSNAPATLTF ENMAGVFMLV AGGIVAGIFL IFIEIAYKRH KDANGAQ UniProtKB: Glutamate receptor ionotropic, NMDA 1 |
-Macromolecule #2: Glutamate receptor
| Macromolecule | Name: Glutamate receptor / type: protein_or_peptide / ID: 2 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 98.888945 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MGTMRLFLLA VLFLFSFARA TGWSHPQFEK GGGSGGGSGG SAWSHPQFEK GALVPRGRSQ KSPPSIGIAV ILVGTSDEVA IKDAHEKDD FHHLSVVPRV ELVAMNETDP KSIITRICDL MSDRKIQGVV FADDTDQEAI AQILDFISAQ TLTPILGIHG G SSMIMADK ...String: MGTMRLFLLA VLFLFSFARA TGWSHPQFEK GGGSGGGSGG SAWSHPQFEK GALVPRGRSQ KSPPSIGIAV ILVGTSDEVA IKDAHEKDD FHHLSVVPRV ELVAMNETDP KSIITRICDL MSDRKIQGVV FADDTDQEAI AQILDFISAQ TLTPILGIHG G SSMIMADK DESSMFFQFG PSIEQQASVM LNIMEEYDWY IFSIVTTYFP GYQDFVNKIR STIENSFVGW ELEEVLLLDM SL DDGDSKI QNQLKKLQSP IILLYCTKEE ATYIFEVANS VGLTGYGYTW IVPSLVAGDT DTVPSEFPTG LISVSYDEWD YGL PARVRD GIAIITTAAS DMLSEHSFIP EPKSSCYNTH EKRIYQSNML NRYLINVTFE GRNLSFSEDG YQMHPKLVII LLNK ERKWE RVGKWKDKSL QMKYYVWPRM CPETEEQEDD HLSIVTLEEA PFVIVESVDP LSGTCMRNTV PCQKRIISEN KTDEE PGYI KKCCKGFCID ILKKISKSVK FTYDLYLVTN GKHGKKINGT WNGMIGEVVM KRAYMAVGSL TINEERSEVV DFSVPF IET GISVMVSRSN GTVSPSAFLE PFSADVWVMM FVMLLIVSAV AVFVFEYFSP VGYNRCLADG REPGGPSFTI GKAIWLL WG LVFNNSVPVQ NPKGTTSKIM VSVWAFFAVI FLASYTANLA AFMIQEEYVD QVSGLSDKKF QRPNDFSPPF RFGTVPNG S TERNIRNNYA EMHAYMGKFN QRGVDDALLS LKTGKLDAFI YDAAVLNYMA GRDEGCKLVT IGSGKVFAST GYGIAIQKD SGWKRQVDLA ILQLFGDGEM EELEALWLTG ICHNEKNEVM SSQLDIDNMA GVFYMLGAAM ALSLITFICE HLFYWQFRHS FMG UniProtKB: Glutamate receptor |
-Macromolecule #3: GLYCINE
| Macromolecule | Name: GLYCINE / type: ligand / ID: 3 / Number of copies: 2 / Formula: GLY |
|---|---|
| Molecular weight | Theoretical: 75.067 Da |
| Chemical component information |  ChemComp-GLY: |
-Macromolecule #4: GLUTAMIC ACID
| Macromolecule | Name: GLUTAMIC ACID / type: ligand / ID: 4 / Number of copies: 2 / Formula: GLU |
|---|---|
| Molecular weight | Theoretical: 147.129 Da |
| Chemical component information |  ChemComp-GLU: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 4 mg/mL |
|---|---|
| Buffer | pH: 7.5 |
| Grid | Model: UltrAuFoil R1.2/1.3 / Material: GOLD / Mesh: 300 / Support film - Material: GOLD / Support film - topology: HOLEY / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 35 sec. |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 85 % / Chamber temperature: 285 K |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 66.3 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.8000000000000003 µm / Nominal defocus min: 1.4000000000000001 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller







 Z
Z Y
Y X
X