+ Open data
Open data
- Basic information
Basic information
| Entry |  | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | L. monocytogenes GS(14)-Q-GlnR peptide | ||||||||||||
 Map data Map data | Sharpened (B factor 110.5) | ||||||||||||
 Sample Sample |
| ||||||||||||
 Keywords Keywords | glutamine synthetase repressor tetradecamer / BIOSYNTHETIC PROTEIN / LIGASE | ||||||||||||
| Function / homology |  Function and homology information Function and homology informationglutamine synthetase / glutamine biosynthetic process / glutamine synthetase activity / regulation of DNA-templated transcription / DNA binding / ATP binding / metal ion binding / cytoplasm Similarity search - Function | ||||||||||||
| Biological species |  Listeria monocytogenes (bacteria) Listeria monocytogenes (bacteria) | ||||||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 2.61 Å | ||||||||||||
 Authors Authors | Travis BA / Peck J | ||||||||||||
| Funding support |  United States, 3 items United States, 3 items
| ||||||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2022 Journal: Nat Commun / Year: 2022Title: Molecular dissection of the glutamine synthetase-GlnR nitrogen regulatory circuitry in Gram-positive bacteria. Authors: Brady A Travis / Jared V Peck / Raul Salinas / Brandon Dopkins / Nicholas Lent / Viet D Nguyen / Mario J Borgnia / Richard G Brennan / Maria A Schumacher /  Abstract: How bacteria sense and respond to nitrogen levels are central questions in microbial physiology. In Gram-positive bacteria, nitrogen homeostasis is controlled by an operon encoding glutamine ...How bacteria sense and respond to nitrogen levels are central questions in microbial physiology. In Gram-positive bacteria, nitrogen homeostasis is controlled by an operon encoding glutamine synthetase (GS), a dodecameric machine that assimilates ammonium into glutamine, and the GlnR repressor. GlnR detects nitrogen excess indirectly by binding glutamine-feedback-inhibited-GS (FBI-GS), which activates its transcription-repression function. The molecular mechanisms behind this regulatory circuitry, however, are unknown. Here we describe biochemical and structural analyses of GS and FBI-GS-GlnR complexes from pathogenic and non-pathogenic Gram-positive bacteria. The structures show FBI-GS binds the GlnR C-terminal domain within its active-site cavity, juxtaposing two GlnR monomers to form a DNA-binding-competent GlnR dimer. The FBI-GS-GlnR interaction stabilizes the inactive GS conformation. Strikingly, this interaction also favors a remarkable dodecamer to tetradecamer transition in some GS, breaking the paradigm that all bacterial GS are dodecamers. These data thus unveil unique structural mechanisms of transcription and enzymatic regulation. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_25866.map.gz emd_25866.map.gz | 229.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-25866-v30.xml emd-25866-v30.xml emd-25866.xml emd-25866.xml | 14.8 KB 14.8 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_25866.png emd_25866.png | 119.3 KB | ||
| Filedesc metadata |  emd-25866.cif.gz emd-25866.cif.gz | 5.7 KB | ||
| Others |  emd_25866_additional_1.map.gz emd_25866_additional_1.map.gz | 121.8 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-25866 http://ftp.pdbj.org/pub/emdb/structures/EMD-25866 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-25866 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-25866 | HTTPS FTP |
-Validation report
| Summary document |  emd_25866_validation.pdf.gz emd_25866_validation.pdf.gz | 579.2 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_25866_full_validation.pdf.gz emd_25866_full_validation.pdf.gz | 578.8 KB | Display | |
| Data in XML |  emd_25866_validation.xml.gz emd_25866_validation.xml.gz | 7.1 KB | Display | |
| Data in CIF |  emd_25866_validation.cif.gz emd_25866_validation.cif.gz | 8.1 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-25866 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-25866 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-25866 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-25866 | HTTPS FTP |
-Related structure data
| Related structure data |  7tf9MC  7tdpC  7tdvC  7teaC  7tecC  7tenC  7tf6C  7tf7C  7tfaC  7tfbC  7tfcC  7tfdC  7tfeC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_25866.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_25866.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Sharpened (B factor 110.5) | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.88 Å | ||||||||||||||||||||||||||||||||||||
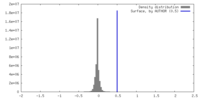
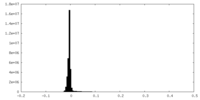
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Additional map: unsharpened
| File | emd_25866_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | unsharpened | ||||||||||||
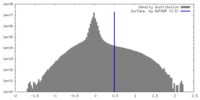
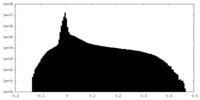
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Tetradecameric L. monocytogenes GS complex with glutamine and Gln...
| Entire | Name: Tetradecameric L. monocytogenes GS complex with glutamine and GlnR C-tail peptides |
|---|---|
| Components |
|
-Supramolecule #1: Tetradecameric L. monocytogenes GS complex with glutamine and Gln...
| Supramolecule | Name: Tetradecameric L. monocytogenes GS complex with glutamine and GlnR C-tail peptides type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#2 |
|---|
-Macromolecule #1: Glutamine synthetase
| Macromolecule | Name: Glutamine synthetase / type: protein_or_peptide / ID: 1 / Number of copies: 14 / Enantiomer: LEVO / EC number: glutamine synthetase |
|---|---|
| Source (natural) | Organism:  Listeria monocytogenes (bacteria) Listeria monocytogenes (bacteria) |
| Molecular weight | Theoretical: 52.763766 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MGSSHHHHHH SSGLVPRGSH MAKYTKEDIF RFADEQNVKF IRLQFTDILG IIKNVEIPVS QLKKALDNKI MFDGSSIEGF VRIEESDMY LFPDLDTWVV FPWTAEKGKV ARMICDIYNP DMTPFAGDPR ANLKRVLKEM EELGFTEFNL GPEPEFFLFK L DENRRPTL ...String: MGSSHHHHHH SSGLVPRGSH MAKYTKEDIF RFADEQNVKF IRLQFTDILG IIKNVEIPVS QLKKALDNKI MFDGSSIEGF VRIEESDMY LFPDLDTWVV FPWTAEKGKV ARMICDIYNP DMTPFAGDPR ANLKRVLKEM EELGFTEFNL GPEPEFFLFK L DENRRPTL ELNDSGGYFD LAPTDLGENC RRDIVLELEE MGFEIEASHH EVAPGQHEID FKYEDAITAC DSIQTFKLVV KT IARKHGL HATFMPKPLF GVNGSGMHFN MSLFNEKGNA FFDESGELEL SQTAYHFLAG MLKHARGYTA VTNPTINSFK RLV PGYEAP CYIAWSGKNR SPLVRVPSSR GLSTRLELRS VDPSANPYLA MAVLLKAGLS GIKDELTPPA PVDRNIYGMN EEER EATGI YDLPESLGHA LIELEKNEII KDGLGEHIFE HFIEAKTIEC DMFRTAVHPW EREQYLEIY UniProtKB: Glutamine synthetase |
-Macromolecule #2: C-tail peptide of Glutamine synthetase repressor
| Macromolecule | Name: C-tail peptide of Glutamine synthetase repressor / type: protein_or_peptide / ID: 2 / Number of copies: 14 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Listeria monocytogenes (bacteria) Listeria monocytogenes (bacteria) |
| Molecular weight | Theoretical: 660.784 Da |
| Sequence | String: QLPRF UniProtKB: Glutamine synthetase repressor |
-Macromolecule #3: MAGNESIUM ION
| Macromolecule | Name: MAGNESIUM ION / type: ligand / ID: 3 / Number of copies: 28 / Formula: MG |
|---|---|
| Molecular weight | Theoretical: 24.305 Da |
-Macromolecule #4: GLUTAMINE
| Macromolecule | Name: GLUTAMINE / type: ligand / ID: 4 / Number of copies: 14 / Formula: GLN |
|---|---|
| Molecular weight | Theoretical: 146.144 Da |
| Chemical component information |  ChemComp-GLN: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.9 mg/mL |
|---|---|
| Buffer | pH: 7.5 |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TALOS ARCTICA |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 42.65 e/Å2 |
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.2 µm / Nominal defocus min: 0.3 µm |
| Experimental equipment |  Model: Talos Arctica / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: NONE |
|---|---|
| Final reconstruction | Applied symmetry - Point group: D7 (2x7 fold dihedral) / Resolution.type: BY AUTHOR / Resolution: 2.61 Å / Resolution method: FSC 0.143 CUT-OFF / Software - Name: cryoSPARC (ver. 3.2) / Number images used: 212272 |
| Initial angle assignment | Type: MAXIMUM LIKELIHOOD / Software - Name: cryoSPARC (ver. 3.2) |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD / Software - Name: cryoSPARC (ver. 3.2) |
-Atomic model buiding 1
| Refinement | Space: REAL |
|---|---|
| Output model |  PDB-7tf9: |
 Movie
Movie Controller
Controller














 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)