[English] 日本語
 Yorodumi
Yorodumi- EMDB-21012: A small terminase protein from a thermophilic phage with a fixed ... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-21012 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | A small terminase protein from a thermophilic phage with a fixed helix-turn-helix geometry, symmetric | |||||||||
 Map data Map data | Cryo-EM reconstruction of the thermophilic bacteriophage P74-26 small terminase- symmetric | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | small terminase / bacteriophage / helix-turn-helix / motor / VIRAL PROTEIN | |||||||||
| Function / homology | : / Phage G20C small terminase, N-terminal domain / Phage G20C small terminase C-terminal domain / Phage G20C small terminase N-terminal domain-containing protein Function and homology information Function and homology information | |||||||||
| Biological species |  Thermus virus P74-26 Thermus virus P74-26 | |||||||||
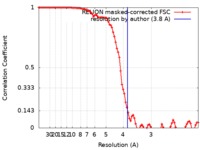
| Method | single particle reconstruction / cryo EM / Resolution: 3.8 Å | |||||||||
 Authors Authors | Hayes JA / Hilbert BJ | |||||||||
| Funding support |  United States, 2 items United States, 2 items
| |||||||||
 Citation Citation |  Journal: J Biol Chem / Year: 2020 Journal: J Biol Chem / Year: 2020Title: A thermophilic phage uses a small terminase protein with a fixed helix-turn-helix geometry. Authors: Janelle A Hayes / Brendan J Hilbert / Christl Gaubitz / Nicholas P Stone / Brian A Kelch /  Abstract: Tailed bacteriophages use a DNA-packaging motor to encapsulate their genome during viral particle assembly. The small terminase (TerS) component of this DNA-packaging machinery acts as a molecular ...Tailed bacteriophages use a DNA-packaging motor to encapsulate their genome during viral particle assembly. The small terminase (TerS) component of this DNA-packaging machinery acts as a molecular matchmaker that recognizes both the viral genome and the main motor component, the large terminase (TerL). However, how TerS binds DNA and the TerL protein remains unclear. Here we identified gp83 of the thermophilic bacteriophage P74-26 as the TerS protein. We found that TerS oligomerizes into a nonamer that binds DNA, stimulates TerL ATPase activity, and inhibits TerL nuclease activity. A cryo-EM structure of TerS revealed that it forms a ring with a wide central pore and radially arrayed helix-turn-helix domains. The structure further showed that these helix-turn-helix domains, which are thought to bind DNA by wrapping the double helix around the ring, are rigidly held in an orientation distinct from that seen in other TerS proteins. This rigid arrangement of the putative DNA-binding domain imposed strong constraints on how TerS can bind DNA. Finally, the TerS structure lacked the conserved C-terminal β-barrel domain used by other TerS proteins for binding TerL. This suggests that a well-ordered C-terminal β-barrel domain is not required for TerS to carry out its matchmaking function. Our work highlights a thermophilic system for studying the role of small terminase proteins in viral maturation and presents the structure of TerS, revealing key differences between this thermophilic phage and its mesophilic counterparts. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_21012.map.gz emd_21012.map.gz | 5.6 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-21012-v30.xml emd-21012-v30.xml emd-21012.xml emd-21012.xml | 18.4 KB 18.4 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_21012_fsc.xml emd_21012_fsc.xml | 9.2 KB | Display |  FSC data file FSC data file |
| Images |  emd_21012.png emd_21012.png | 75.9 KB | ||
| Filedesc metadata |  emd-21012.cif.gz emd-21012.cif.gz | 5.7 KB | ||
| Others |  emd_21012_half_map_1.map.gz emd_21012_half_map_1.map.gz emd_21012_half_map_2.map.gz emd_21012_half_map_2.map.gz | 49.6 MB 49.5 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-21012 http://ftp.pdbj.org/pub/emdb/structures/EMD-21012 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-21012 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-21012 | HTTPS FTP |
-Validation report
| Summary document |  emd_21012_validation.pdf.gz emd_21012_validation.pdf.gz | 557.3 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_21012_full_validation.pdf.gz emd_21012_full_validation.pdf.gz | 556.9 KB | Display | |
| Data in XML |  emd_21012_validation.xml.gz emd_21012_validation.xml.gz | 16.2 KB | Display | |
| Data in CIF |  emd_21012_validation.cif.gz emd_21012_validation.cif.gz | 21.3 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-21012 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-21012 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-21012 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-21012 | HTTPS FTP |
-Related structure data
| Related structure data |  6v1iMC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_21012.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_21012.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Cryo-EM reconstruction of the thermophilic bacteriophage P74-26 small terminase- symmetric | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.059 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
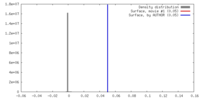
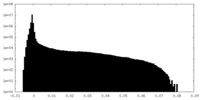
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Half map: Half map 1 of the 9-fold symmetric cryoEM...
| File | emd_21012_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map 1 of the 9-fold symmetric cryoEM reconstruction of the small terminase protein from the thermophilic bacteriophage P74-26. | ||||||||||||
| Projections & Slices |
| ||||||||||||
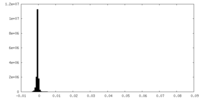
| Density Histograms |
-Half map: Half map 2 of the 9-fold symmetric cryoEM...
| File | emd_21012_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map 2 of the 9-fold symmetric cryoEM reconstruction of the small terminase protein from the thermophilic bacteriophage P74-26. | ||||||||||||
| Projections & Slices |
| ||||||||||||
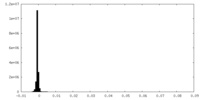
| Density Histograms |
- Sample components
Sample components
-Entire : Gene product 83 of the Thermus thermophilus bacteriophage P74-26
| Entire | Name: Gene product 83 of the Thermus thermophilus bacteriophage P74-26 |
|---|---|
| Components |
|
-Supramolecule #1: Gene product 83 of the Thermus thermophilus bacteriophage P74-26
| Supramolecule | Name: Gene product 83 of the Thermus thermophilus bacteriophage P74-26 type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  Thermus virus P74-26 Thermus virus P74-26 |
| Molecular weight | Theoretical: 171 KDa |
-Macromolecule #1: Small terminase protein
| Macromolecule | Name: Small terminase protein / type: protein_or_peptide / ID: 1 / Number of copies: 9 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Thermus virus P74-26 Thermus virus P74-26 |
| Molecular weight | Theoretical: 19.080887 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: GPHMSVSFRD RVLKLYLLGF DPSEIAQTLS LDAKRKVTEE EVLHVLAEAR ELLSALPSLE DIRAEVGQAL ERARIFQKDL LAIYQNMLR NYNAMMEGLT EHPDGTPVIG VRPADIAAMA DRIMKIDQER ITALLNSLKV LGHVGSTTAG ALPSATELVS V EELVAEVA DETPKT UniProtKB: Phage G20C small terminase N-terminal domain-containing protein |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 3.4 mg/mL | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.5 Component:
| ||||||||||||
| Grid | Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 60 sec. / Pretreatment - Atmosphere: OTHER / Details: 20 mA | ||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 95 % / Chamber temperature: 283 K / Instrument: FEI VITROBOT MARK IV | ||||||||||||
| Details | This sample was monodisperse (PDI= 1.000) |
- Electron microscopy
Electron microscopy
| Microscope | TFS KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: SUPER-RESOLUTION / Number grids imaged: 2 / Number real images: 2822 / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Calibrated defocus max: 2.6 µm / Calibrated defocus min: 1.4000000000000001 µm / Calibrated magnification: 13000 / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm |
| Sample stage | Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Initial model | PDB ID: Chain - Chain ID: A / Chain - Residue range: 1-137 / Chain - Source name: PDB / Chain - Initial model type: experimental model |
|---|---|
| Refinement | Space: REAL / Protocol: FLEXIBLE FIT |
| Output model |  PDB-6v1i: |
 Movie
Movie Controller
Controller











 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)