[English] 日本語
 Yorodumi
Yorodumi- EMDB-17943: Structure of the immature HTLV-1 CA lattice from full-length Gag ... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of the immature HTLV-1 CA lattice from full-length Gag VLPs: CA-CTD refinement | |||||||||||||||
 Map data Map data | HTLV-1 Gag-based VLPs, CA-CTD refinement, B-factor sharpened map | |||||||||||||||
 Sample Sample |
| |||||||||||||||
 Keywords Keywords | Retrovirus / HTLV / immature capsid / CA / VIRAL PROTEIN | |||||||||||||||
| Function / homology |  Function and homology information Function and homology informationviral process / viral nucleocapsid / nucleic acid binding / structural molecule activity / zinc ion binding Similarity search - Function | |||||||||||||||
| Biological species |  Human T-cell leukemia virus type I Human T-cell leukemia virus type I | |||||||||||||||
| Method | subtomogram averaging / cryo EM / Resolution: 6.2 Å | |||||||||||||||
 Authors Authors | Obr M / Percipalle M / Chernikova D / Yang H / Thader A / Pinke G / Porley D / Mansky LM / Dick RA / Schur FKM | |||||||||||||||
| Funding support |  Austria, Austria,  United States, 4 items United States, 4 items
| |||||||||||||||
 Citation Citation |  Journal: bioRxiv / Year: 2023 Journal: bioRxiv / Year: 2023Title: Unconventional stabilization of the human T-cell leukemia virus type 1 immature Gag lattice. Authors: Martin Obr / Mathias Percipalle / Darya Chernikova / Huixin Yang / Andreas Thader / Gergely Pinke / Dario Porley / Louis M Mansky / Robert A Dick / Florian Km Schur /   Abstract: Human T-cell leukemia virus type 1 (HTLV-1) has an atypical immature particle morphology compared to other retroviruses. This indicates that these particles are formed in a way that is unique. Here ...Human T-cell leukemia virus type 1 (HTLV-1) has an atypical immature particle morphology compared to other retroviruses. This indicates that these particles are formed in a way that is unique. Here we report the results of cryo-electron tomography (cryo-ET) studies of HTLV-1 virus-like particles (VLPs) assembled , as well as derived from cells. This work shows that HTLV-1 employs an unconventional mechanism of Gag-Gag interactions to form the immature viral lattice. Analysis of high-resolution structural information from immature CA tubular arrays reveals that the primary stabilizing component in HTLV-1 is CA-NTD. Mutagenesis and biophysical analysis support this observation. This distinguishes HTLV-1 from other retroviruses, in which the stabilization is provided primarily by the CA-CTD. These results are the first to provide structural details of the quaternary arrangement of Gag for an immature deltaretrovirus, and this helps explain why HTLV-1 particles are morphologically distinct. | |||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_17943.map.gz emd_17943.map.gz | 116.2 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-17943-v30.xml emd-17943-v30.xml emd-17943.xml emd-17943.xml | 25.4 KB 25.4 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_17943.png emd_17943.png | 68.4 KB | ||
| Others |  emd_17943_additional_1.map.gz emd_17943_additional_1.map.gz emd_17943_half_map_1.map.gz emd_17943_half_map_1.map.gz emd_17943_half_map_2.map.gz emd_17943_half_map_2.map.gz | 108.7 MB 64.1 MB 64.1 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-17943 http://ftp.pdbj.org/pub/emdb/structures/EMD-17943 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-17943 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-17943 | HTTPS FTP |
-Validation report
| Summary document |  emd_17943_validation.pdf.gz emd_17943_validation.pdf.gz | 1.1 MB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_17943_full_validation.pdf.gz emd_17943_full_validation.pdf.gz | 1.1 MB | Display | |
| Data in XML |  emd_17943_validation.xml.gz emd_17943_validation.xml.gz | 13.8 KB | Display | |
| Data in CIF |  emd_17943_validation.cif.gz emd_17943_validation.cif.gz | 16.3 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-17943 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-17943 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-17943 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-17943 | HTTPS FTP |
-Related structure data
| Related structure data |  8puhMC  8pu6C  8pu7C  8pu8C  8pu9C  8puaC  8pubC  8pucC  8pudC  8pueC  8pufC  8pugC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_17943.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_17943.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | HTLV-1 Gag-based VLPs, CA-CTD refinement, B-factor sharpened map | ||||||||||||||||||||
| Voxel size | X=Y=Z: 1.381 Å | ||||||||||||||||||||
| Density |
| ||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Additional map: HTLV-1 Gag-based VLPs, CA-CTD refinement, denoised map
| File | emd_17943_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | HTLV-1 Gag-based VLPs, CA-CTD refinement, denoised map | ||||||||||||
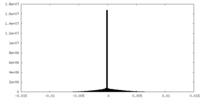
| Projections & Slices |
| ||||||||||||
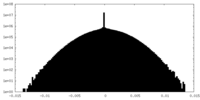
| Density Histograms |
-Half map: HTLV-1 Gag-based VLPs, CA-CTD refinement, halfmap 2
| File | emd_17943_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | HTLV-1 Gag-based VLPs, CA-CTD refinement, halfmap 2 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: HTLV-1 Gag-based VLPs, CA-CTD refinement, halfmap 1
| File | emd_17943_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | HTLV-1 Gag-based VLPs, CA-CTD refinement, halfmap 1 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Human T-cell leukemia virus type I
| Entire | Name:  Human T-cell leukemia virus type I Human T-cell leukemia virus type I |
|---|---|
| Components |
|
-Supramolecule #1: Human T-cell leukemia virus type I
| Supramolecule | Name: Human T-cell leukemia virus type I / type: virus / ID: 1 / Parent: 0 / Macromolecule list: all / NCBI-ID: 11908 / Sci species name: Human T-cell leukemia virus type I / Virus type: VIRUS-LIKE PARTICLE / Virus isolate: STRAIN / Virus enveloped: Yes / Virus empty: Yes |
|---|
-Macromolecule #1: Gag polyprotein
| Macromolecule | Name: Gag polyprotein / type: protein_or_peptide / ID: 1 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Human T-cell leukemia virus type I Human T-cell leukemia virus type I |
| Molecular weight | Theoretical: 47.553234 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MGQIFSRSAS PIPRPPRGLA AHHWLNFLQA AYRLEPGPSS YDFHQLKKFL KIALETPARI CPINYSLLAS LLPKGYPGRV NEILHILIQ TQAQIPSRPA PPPPSSPTHD PPDSDPQIPP PYVEPTAPQV LPVMHPHGAP PNHRPWQMKD LQAIKQEVSQ A APGSPQFM ...String: MGQIFSRSAS PIPRPPRGLA AHHWLNFLQA AYRLEPGPSS YDFHQLKKFL KIALETPARI CPINYSLLAS LLPKGYPGRV NEILHILIQ TQAQIPSRPA PPPPSSPTHD PPDSDPQIPP PYVEPTAPQV LPVMHPHGAP PNHRPWQMKD LQAIKQEVSQ A APGSPQFM QTIRLAVQQF DPTAKDLQDL LQYLCSSLVA SLHHQQLDSL ISEAETRGIT GYNPLAGPLR VQANNPQQQG LR REYQQLW LAAFAALPGS AKDPSWASIL QGLEEPYHAF VERLNIALDN GLPEGTPKDP ILRSLAYSNA NKECQKLLQA RGH TNSPLG DMLRACQTWT PKDKTKVLVV QPKKPPPNQP CFRCGKAGHW SRDCTQPRPP PGPCPLCQDP THWKRDCPRL KPTI PEPEP EEDALLLDLP ADIPHPKNSI GGEV UniProtKB: Gag polyprotein |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | subtomogram averaging |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 Component:
Details: Phosphate-buffered saline (PBS) 1X | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grid | Model: C-flat-2/2 / Material: COPPER / Mesh: 300 / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 120 sec. / Pretreatment - Atmosphere: AIR | ||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 90 % / Chamber temperature: 283 K / Instrument: LEICA EM GP |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Specialist optics | Energy filter - Name: GIF Bioquantum / Energy filter - Slit width: 20 eV |
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Digitization - Dimensions - Width: 5760 pixel / Digitization - Dimensions - Height: 4092 pixel / Average exposure time: 0.32 sec. / Average electron dose: 3.5 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 4.0 µm / Nominal defocus min: 1.25 µm / Nominal magnification: 80000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Final reconstruction | Number classes used: 1 / Applied symmetry - Point group: C2 (2 fold cyclic) / Algorithm: BACK PROJECTION / Resolution.type: BY AUTHOR / Resolution: 6.2 Å / Resolution method: FSC 0.143 CUT-OFF / Software - Name: Warp (ver. 1.0.9) / Software - details: Refinement and reconstruction in M / Number subtomograms used: 29000 |
|---|---|
| Extraction | Number tomograms: 85 / Number images used: 132000 Software: (Name: subTOM, Warp (ver. 1.0.9),  MATLAB (ver. R2018b)) MATLAB (ver. R2018b)) |
| Final 3D classification | Number classes: 5 / Software - Name: RELION (ver. 3.0.8) |
| Final angle assignment | Type: PROJECTION MATCHING / Software - Name: Warp (ver. 1.0.9) / Software - details: Multiparticle refinement in M |
-Atomic model buiding 1
| Initial model | Chain - Residue range: 13-125 / Chain - Source name: AlphaFold / Chain - Initial model type: in silico model / Details: rigid body fit using AlphaFold derived model |
|---|---|
| Refinement | Space: REAL / Protocol: RIGID BODY FIT |
| Output model |  PDB-8puh: |
 Movie
Movie Controller
Controller


















 Z
Z Y
Y X
X