+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-13961 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of the B. subtilis disome - collided 70S ribosome | |||||||||
 Map data Map data | disome map after focused refinement on collided ribosome | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Ribosome rescue / disome / ribosome collision / stalling / no-go complex / RIBOSOME | |||||||||
| Function / homology |  Function and homology information Function and homology informationlarge ribosomal subunit / small ribosomal subunit / large ribosomal subunit rRNA binding / transferase activity / ribosomal large subunit assembly / cytosolic large ribosomal subunit / tRNA binding / rRNA binding / ribosome / structural constituent of ribosome ...large ribosomal subunit / small ribosomal subunit / large ribosomal subunit rRNA binding / transferase activity / ribosomal large subunit assembly / cytosolic large ribosomal subunit / tRNA binding / rRNA binding / ribosome / structural constituent of ribosome / ribonucleoprotein complex / translation / mRNA binding / zinc ion binding / cytoplasm Similarity search - Function | |||||||||
| Biological species |  | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 5.45 Å | |||||||||
 Authors Authors | Kratzat H / Buschauer R | |||||||||
| Funding support |  Germany, 1 items Germany, 1 items
| |||||||||
 Citation Citation |  Journal: Nature / Year: 2022 Journal: Nature / Year: 2022Title: Ribosome collisions induce mRNA cleavage and ribosome rescue in bacteria. Authors: Kazuki Saito / Hanna Kratzat / Annabelle Campbell / Robert Buschauer / A Maxwell Burroughs / Otto Berninghausen / L Aravind / Rachel Green / Roland Beckmann / Allen R Buskirk /   Abstract: Ribosome rescue pathways recycle stalled ribosomes and target problematic mRNAs and aborted proteins for degradation. In bacteria, it remains unclear how rescue pathways distinguish ribosomes stalled ...Ribosome rescue pathways recycle stalled ribosomes and target problematic mRNAs and aborted proteins for degradation. In bacteria, it remains unclear how rescue pathways distinguish ribosomes stalled in the middle of a transcript from actively translating ribosomes. Here, using a genetic screen in Escherichia coli, we discovered a new rescue factor that has endonuclease activity. SmrB cleaves mRNAs upstream of stalled ribosomes, allowing the ribosome rescue factor tmRNA (which acts on truncated mRNAs) to rescue upstream ribosomes. SmrB is recruited to ribosomes and is activated by collisions. Cryo-electron microscopy structures of collided disomes from E. coli and Bacillus subtilis show distinct and conserved arrangements of individual ribosomes and the composite SmrB-binding site. These findings reveal the underlying mechanisms by which ribosome collisions trigger ribosome rescue in bacteria. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_13961.map.gz emd_13961.map.gz | 407.3 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-13961-v30.xml emd-13961-v30.xml emd-13961.xml emd-13961.xml | 66.4 KB 66.4 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_13961.png emd_13961.png | 81.3 KB | ||
| Filedesc metadata |  emd-13961.cif.gz emd-13961.cif.gz | 12.6 KB | ||
| Others |  emd_13961_additional_1.map.gz emd_13961_additional_1.map.gz emd_13961_additional_2.map.gz emd_13961_additional_2.map.gz | 450.5 MB 407.1 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-13961 http://ftp.pdbj.org/pub/emdb/structures/EMD-13961 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-13961 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-13961 | HTTPS FTP |
-Validation report
| Summary document |  emd_13961_validation.pdf.gz emd_13961_validation.pdf.gz | 537.6 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_13961_full_validation.pdf.gz emd_13961_full_validation.pdf.gz | 537.1 KB | Display | |
| Data in XML |  emd_13961_validation.xml.gz emd_13961_validation.xml.gz | 8.8 KB | Display | |
| Data in CIF |  emd_13961_validation.cif.gz emd_13961_validation.cif.gz | 10.2 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-13961 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-13961 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-13961 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-13961 | HTTPS FTP |
-Related structure data
| Related structure data |  7qh4MC  7qg8C  7qghC  7qgnC  7qgrC  7qguC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_13961.map.gz / Format: CCP4 / Size: 824 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_13961.map.gz / Format: CCP4 / Size: 824 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | disome map after focused refinement on collided ribosome | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.084 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
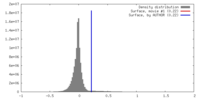
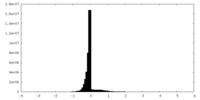
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Additional map: local resolution filtered map
| File | emd_13961_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | local resolution filtered map | ||||||||||||
| Projections & Slices |
| ||||||||||||
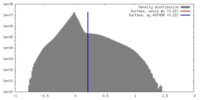
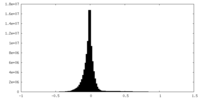
| Density Histograms |
-Additional map: disome map
| File | emd_13961_additional_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | disome map | ||||||||||||
| Projections & Slices |
| ||||||||||||
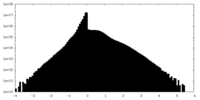
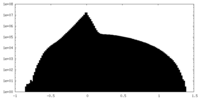
| Density Histograms |
- Sample components
Sample components
+Entire : collided ribosome of the disome structure
+Supramolecule #1: collided ribosome of the disome structure
+Macromolecule #1: 23S rRNA
+Macromolecule #2: 5S rRNA
+Macromolecule #31: 16S rRNA
+Macromolecule #51: tRNA
+Macromolecule #3: 50S ribosomal protein L2
+Macromolecule #4: 50S ribosomal protein L3
+Macromolecule #5: 50S ribosomal protein L4
+Macromolecule #6: 50S ribosomal protein L5
+Macromolecule #7: Ribosomal protein L6
+Macromolecule #8: 50S ribosomal protein L10
+Macromolecule #9: 50S ribosomal protein L11
+Macromolecule #10: 50S ribosomal protein L13
+Macromolecule #11: 50S ribosomal protein L14
+Macromolecule #12: 50S ribosomal protein L15
+Macromolecule #13: 50S ribosomal protein L16
+Macromolecule #14: 50S ribosomal protein L17
+Macromolecule #15: 50S ribosomal protein L18
+Macromolecule #16: 50S ribosomal protein L19
+Macromolecule #17: 50S ribosomal protein L20
+Macromolecule #18: 50S ribosomal protein L21
+Macromolecule #19: 50S ribosomal protein L22
+Macromolecule #20: 50S ribosomal protein L23
+Macromolecule #21: 50S ribosomal protein L24
+Macromolecule #22: 50S ribosomal protein L27
+Macromolecule #23: 50S ribosomal protein L28
+Macromolecule #24: 50S ribosomal protein L29
+Macromolecule #25: 50S ribosomal protein L30
+Macromolecule #26: 50S ribosomal protein L32
+Macromolecule #27: 50S ribosomal protein L33
+Macromolecule #28: 50S ribosomal protein L34
+Macromolecule #29: 50S ribosomal protein L35
+Macromolecule #30: 50S ribosomal protein L36
+Macromolecule #32: 30S ribosomal protein S2
+Macromolecule #33: 30S ribosomal protein S3
+Macromolecule #34: 30S ribosomal protein S4
+Macromolecule #35: 30S ribosomal protein S5
+Macromolecule #36: 30S ribosomal protein S6
+Macromolecule #37: 30S ribosomal protein S7
+Macromolecule #38: 30S ribosomal protein S8
+Macromolecule #39: 30S ribosomal protein S9
+Macromolecule #40: 30S ribosomal protein S10
+Macromolecule #41: 30S ribosomal protein S11
+Macromolecule #42: 30S ribosomal protein S12
+Macromolecule #43: 30S ribosomal protein S13
+Macromolecule #44: 30S ribosomal protein S14 type Z
+Macromolecule #45: 30S ribosomal protein S15
+Macromolecule #46: 30S ribosomal protein S16
+Macromolecule #47: 30S ribosomal protein S17
+Macromolecule #48: 30S ribosomal protein S18
+Macromolecule #49: 30S ribosomal protein S19
+Macromolecule #50: 30S ribosomal protein S20
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: FEI FALCON II (4k x 4k) / Average electron dose: 40.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 4.0 µm / Nominal defocus min: 0.5 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: PDB ENTRY PDB model - PDB ID: |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 5.45 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 12739 |
| Initial angle assignment | Type: MAXIMUM LIKELIHOOD |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
 Movie
Movie Controller
Controller





























 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)