+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-13637 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | In-situ structure of hexameric S-layer protein | ||||||||||||
 Map data Map data | Postprocessed map without B-factor sharpening. | ||||||||||||
 Sample Sample |
| ||||||||||||
| Function / homology | Surface glycoprotein signal peptide / Major cell surface glycoprotein / PGF-CTERM archaeal protein-sorting signal / PGF-CTERM motif / S-layer / cell wall organization / extracellular region / plasma membrane / Cell surface glycoprotein Function and homology information Function and homology information | ||||||||||||
| Biological species |  Haloferax volcanii DS2 (archaea) Haloferax volcanii DS2 (archaea) | ||||||||||||
| Method | subtomogram averaging / cryo EM / Resolution: 7.968 Å | ||||||||||||
 Authors Authors | von Kuegelgen A / Bharat TAM | ||||||||||||
| Funding support |  United Kingdom, 3 items United Kingdom, 3 items
| ||||||||||||
 Citation Citation |  Journal: Cell Rep / Year: 2021 Journal: Cell Rep / Year: 2021Title: Complete atomic structure of a native archaeal cell surface. Authors: Andriko von Kügelgen / Vikram Alva / Tanmay A M Bharat /   Abstract: Many prokaryotic cells are covered by an ordered, proteinaceous, sheet-like structure called a surface layer (S-layer). S-layer proteins (SLPs) are usually the highest copy number macromolecules in ...Many prokaryotic cells are covered by an ordered, proteinaceous, sheet-like structure called a surface layer (S-layer). S-layer proteins (SLPs) are usually the highest copy number macromolecules in prokaryotes, playing critical roles in cellular physiology such as blocking predators, scaffolding membranes, and facilitating environmental interactions. Using electron cryomicroscopy of two-dimensional sheets, we report the atomic structure of the S-layer from the archaeal model organism Haloferax volcanii. This S-layer consists of a hexagonal array of tightly interacting immunoglobulin-like domains, which are also found in SLPs across several classes of archaea. Cellular tomography reveal that the S-layer is nearly continuous on the cell surface, completed by pentameric defects in the hexagonal lattice. We further report the atomic structure of the SLP pentamer, which shows markedly different relative arrangements of SLP domains needed to complete the S-layer. Our structural data provide a framework for understanding cell surfaces of archaea at the atomic level. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_13637.map.gz emd_13637.map.gz | 24 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-13637-v30.xml emd-13637-v30.xml emd-13637.xml emd-13637.xml | 19.8 KB 19.8 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_13637.png emd_13637.png | 159.9 KB | ||
| Masks |  emd_13637_msk_1.map emd_13637_msk_1.map | 27 MB |  Mask map Mask map | |
| Others |  emd_13637_additional_1.map.gz emd_13637_additional_1.map.gz | 19.4 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-13637 http://ftp.pdbj.org/pub/emdb/structures/EMD-13637 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-13637 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-13637 | HTTPS FTP |
-Validation report
| Summary document |  emd_13637_validation.pdf.gz emd_13637_validation.pdf.gz | 372.8 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_13637_full_validation.pdf.gz emd_13637_full_validation.pdf.gz | 372.4 KB | Display | |
| Data in XML |  emd_13637_validation.xml.gz emd_13637_validation.xml.gz | 5.7 KB | Display | |
| Data in CIF |  emd_13637_validation.cif.gz emd_13637_validation.cif.gz | 6.4 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-13637 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-13637 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-13637 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-13637 | HTTPS FTP |
-Related structure data
| Related structure data |  7pttMC  7ptpC  7ptrC  7ptuC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_13637.map.gz / Format: CCP4 / Size: 27 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_13637.map.gz / Format: CCP4 / Size: 27 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Postprocessed map without B-factor sharpening. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.328 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
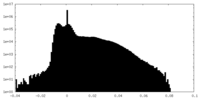
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Mask #1
| File |  emd_13637_msk_1.map emd_13637_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
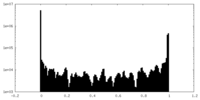
| Density Histograms |
-Additional map: Full map without postprocessing or B-factor sharpening
| File | emd_13637_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Full map without postprocessing or B-factor sharpening | ||||||||||||
| Projections & Slices |
| ||||||||||||
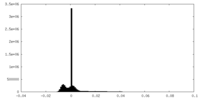
| Density Histograms |
- Sample components
Sample components
-Entire : In-situ structure of hexameric S-layer of Haloferax volcanii
| Entire | Name: In-situ structure of hexameric S-layer of Haloferax volcanii |
|---|---|
| Components |
|
-Supramolecule #1: In-situ structure of hexameric S-layer of Haloferax volcanii
| Supramolecule | Name: In-situ structure of hexameric S-layer of Haloferax volcanii type: organelle_or_cellular_component / ID: 1 / Parent: 0 / Macromolecule list: all Details: In-situ structure of hexameric S-layer of Haloferax volcanii |
|---|---|
| Source (natural) | Organism:  Haloferax volcanii DS2 (archaea) / Location in cell: Cell surface Haloferax volcanii DS2 (archaea) / Location in cell: Cell surface |
-Macromolecule #1: Cell surface glycoprotein
| Macromolecule | Name: Cell surface glycoprotein / type: protein_or_peptide / ID: 1 / Number of copies: 6 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Haloferax volcanii DS2 (archaea) Haloferax volcanii DS2 (archaea) |
| Molecular weight | Theoretical: 81.755602 KDa |
| Sequence | String: ERGNLDADSE SFNKTIQSGD RVFLGEEIST DAGLGASNPL LTGTAGNSEG VSLDLSSPIP QTTENQPLGT YDVDGSGSAT TPNVTLLAP RITDSEILTS SGGDVTGSAI SSSDAGNLYV NADYNYESAE KVEVTVEDPS GTDITNEVLS GTDTFVDDGS I GSTSSTGG ...String: ERGNLDADSE SFNKTIQSGD RVFLGEEIST DAGLGASNPL LTGTAGNSEG VSLDLSSPIP QTTENQPLGT YDVDGSGSAT TPNVTLLAP RITDSEILTS SGGDVTGSAI SSSDAGNLYV NADYNYESAE KVEVTVEDPS GTDITNEVLS GTDTFVDDGS I GSTSSTGG GVGIDMSDQD AGEYTIILEG AEDLDFGDAT ETMTLTISSQ DEIGIELDSE SVTQGTDVQY TVTNGIDGNE HV VAMDLSD LQNDATTEQA KEVFRNIGDT SEVGIANSSA TNTSGSSTGP TVETADIAYA VVEIDGASAV GGIETQYLDD SEV DLEVYD AGVSATAAVG QDATNDITLT IEEGGTTLSS PTGQYVVGSE VDINGTATSS DSVAIYVRDD GDWQLLEIGG DNEI SVDSD DTFEEEDIAL SGLSGDGSSI LSLTGTYRIG VIDASDADVG GDGSVDDSLT TSEFTSGVSS SNSIRVTDQA LTGQF TTIN GQVAPVETGT VDINGTASGA NSVLVIFVDE RGNVNYQEVS VDSDGTYDED DITVGLTQGR VTAHILSVGR DSAIGD GSL PSGPSNGATL NDLTGYLDTL DQNNNNGEQI NELIASETVD ETASDDLIVT ETFRLAESST SIDSIYPDAA EAAGINP VA TGETMVIAGS TNLKPDDNTI SIEVTNEDGT SVALEDTDEW NNDGQWMVEI DTTDFETGTF TVEADDGDNT DTVNVEVV S EREDTTTSSD NATDTTTTTD GPTETTTTAE PTETTEEPTE ETTTSSNTPG FGIAVALVAL VGAALLALRR EN |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | subtomogram averaging |
| Aggregation state | cell |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 Component:
Details: 18 % (w/v) artificial sea water | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grid | Model: Quantifoil R2/2 / Material: COPPER/RHODIUM / Mesh: 200 / Support film - Material: CARBON / Support film - topology: HOLEY ARRAY / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Atmosphere: AIR / Details: 20 seconds, 15 mA | |||||||||||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 283.15 K / Instrument: FEI VITROBOT MARK IV Details: Vitrobot options: Blot time 2.0 seconds, Blot force -15,1, Wait time 0 seconds, Drain time 0.5 seconds. | |||||||||||||||||||||
| Details | Haloferax volcanii vesicles |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Temperature | Min: 70.0 K / Max: 70.0 K |
| Specialist optics | Spherical aberration corrector: not used / Chromatic aberration corrector: not used / Energy filter - Name: GIF Quantum LS / Energy filter - Slit width: 20 eV |
| Details | SerialEM Hagen Scheme |
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: COUNTING / Digitization - Dimensions - Width: 3838 pixel / Digitization - Dimensions - Height: 3710 pixel / Number grids imaged: 1 / Average exposure time: 1.0 sec. / Average electron dose: 2.9 e/Å2 / Details: Dose symmetric tilt scheme (Hagen et al, JSB) |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 50.0 µm / Calibrated defocus max: 4.0 µm / Calibrated defocus min: 1.0 µm / Calibrated magnification: 105000 / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 4.0 µm / Nominal defocus min: 1.0 µm / Nominal magnification: 105000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Details | Rigid body fit inside coot of D1-D6 domains and real space refinement with restraints of the original model obtained by single particle analysis in PHENIX. |
|---|---|
| Refinement | Space: REAL / Protocol: RIGID BODY FIT / Target criteria: Best Fit |
| Output model |  PDB-7ptt: |
 Movie
Movie Controller
Controller












 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)