+ データを開く
データを開く
- 基本情報
基本情報
| 登録情報 | データベース: EMDB / ID: EMD-0586 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| タイトル | Cryo-EM structure of the centromeric nucleosome with native alpha satellite DNA | |||||||||
 マップデータ マップデータ | Main EM map after sharpening | |||||||||
 試料 試料 |
| |||||||||
 キーワード キーワード | CENP-A / Centromere / Native alpha satellite DNA / nucleosome / NUCLEAR PROTEIN | |||||||||
| 機能・相同性 |  機能・相同性情報 機能・相同性情報CENP-A containing chromatin assembly / kinetochore assembly / protein localization to chromosome, centromeric region / condensed chromosome, centromeric region / establishment of mitotic spindle orientation / mitotic cytokinesis / chromosome, centromeric region / negative regulation of tumor necrosis factor-mediated signaling pathway / pericentric heterochromatin / negative regulation of megakaryocyte differentiation ...CENP-A containing chromatin assembly / kinetochore assembly / protein localization to chromosome, centromeric region / condensed chromosome, centromeric region / establishment of mitotic spindle orientation / mitotic cytokinesis / chromosome, centromeric region / negative regulation of tumor necrosis factor-mediated signaling pathway / pericentric heterochromatin / negative regulation of megakaryocyte differentiation / protein localization to CENP-A containing chromatin / Replacement of protamines by nucleosomes in the male pronucleus / CENP-A containing nucleosome / Packaging Of Telomere Ends / Amplification of signal from unattached kinetochores via a MAD2 inhibitory signal / Recognition and association of DNA glycosylase with site containing an affected purine / Cleavage of the damaged purine / Deposition of new CENPA-containing nucleosomes at the centromere / Mitotic Prometaphase / telomere organization / Recognition and association of DNA glycosylase with site containing an affected pyrimidine / Cleavage of the damaged pyrimidine / EML4 and NUDC in mitotic spindle formation / RNA Polymerase I Promoter Opening / Inhibition of DNA recombination at telomere / Assembly of the ORC complex at the origin of replication / Meiotic synapsis / SUMOylation of chromatin organization proteins / Regulation of endogenous retroelements by the Human Silencing Hub (HUSH) complex / Resolution of Sister Chromatid Cohesion / DNA methylation / Condensation of Prophase Chromosomes / Chromatin modifications during the maternal to zygotic transition (MZT) / SIRT1 negatively regulates rRNA expression / HCMV Late Events / ERCC6 (CSB) and EHMT2 (G9a) positively regulate rRNA expression / PRC2 methylates histones and DNA / innate immune response in mucosa / Regulation of endogenous retroelements by KRAB-ZFP proteins / Defective pyroptosis / HDACs deacetylate histones / Regulation of endogenous retroelements by Piwi-interacting RNAs (piRNAs) / RNA Polymerase I Promoter Escape / Nonhomologous End-Joining (NHEJ) / lipopolysaccharide binding / Transcriptional regulation by small RNAs / RHO GTPases Activate Formins / Formation of the beta-catenin:TCF transactivating complex / Activated PKN1 stimulates transcription of AR (androgen receptor) regulated genes KLK2 and KLK3 / RUNX1 regulates genes involved in megakaryocyte differentiation and platelet function / HDMs demethylate histones / G2/M DNA damage checkpoint / NoRC negatively regulates rRNA expression / B-WICH complex positively regulates rRNA expression / PKMTs methylate histone lysines / DNA Damage/Telomere Stress Induced Senescence / Pre-NOTCH Transcription and Translation / Meiotic recombination / Activation of anterior HOX genes in hindbrain development during early embryogenesis / Metalloprotease DUBs / RMTs methylate histone arginines / Transcriptional regulation of granulopoiesis / HCMV Early Events / antimicrobial humoral immune response mediated by antimicrobial peptide / structural constituent of chromatin / Separation of Sister Chromatids / UCH proteinases / antibacterial humoral response / heterochromatin formation / nucleosome / nucleosome assembly / E3 ubiquitin ligases ubiquitinate target proteins / Recruitment and ATM-mediated phosphorylation of repair and signaling proteins at DNA double strand breaks / HATs acetylate histones / RUNX1 regulates transcription of genes involved in differentiation of HSCs / MLL4 and MLL3 complexes regulate expression of PPARG target genes in adipogenesis and hepatic steatosis / chromatin organization / Processing of DNA double-strand break ends / Senescence-Associated Secretory Phenotype (SASP) / Oxidative Stress Induced Senescence / defense response to Gram-negative bacterium / Estrogen-dependent gene expression / killing of cells of another organism / chromosome, telomeric region / defense response to Gram-positive bacterium / Ub-specific processing proteases / protein heterodimerization activity / Amyloid fiber formation / negative regulation of cell population proliferation / chromatin binding / protein-containing complex / extracellular space / DNA binding / RNA binding / extracellular exosome / extracellular region / nucleoplasm / nucleus / membrane / cytosol 類似検索 - 分子機能 | |||||||||
| 生物種 |  Homo sapiens (ヒト) Homo sapiens (ヒト) | |||||||||
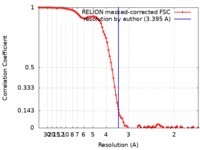
| 手法 | 単粒子再構成法 / クライオ電子顕微鏡法 / 解像度: 3.395 Å | |||||||||
 データ登録者 データ登録者 | Zhou B-R | |||||||||
| 資金援助 |  米国, 1件 米国, 1件
| |||||||||
 引用 引用 |  ジャーナル: Nat Commun / 年: 2019 ジャーナル: Nat Commun / 年: 2019タイトル: Atomic resolution cryo-EM structure of a native-like CENP-A nucleosome aided by an antibody fragment. 著者: Bing-Rui Zhou / K N Sathish Yadav / Mario Borgnia / Jingjun Hong / Baohua Cao / Ada L Olins / Donald E Olins / Yawen Bai / Ping Zhang /  要旨: Genomic DNA in eukaryotes is organized into chromatin through association with core histones to form nucleosomes, each distinguished by their DNA sequences and histone variants. Here, we used a ...Genomic DNA in eukaryotes is organized into chromatin through association with core histones to form nucleosomes, each distinguished by their DNA sequences and histone variants. Here, we used a single-chain antibody fragment (scFv) derived from the anti-nucleosome antibody mAb PL2-6 to stabilize human CENP-A nucleosome containing a native α-satellite DNA and solved its structure by the cryo-electron microscopy (cryo-EM) to 2.6 Å resolution. In comparison, the corresponding cryo-EM structure of the free CENP-A nucleosome could only reach 3.4 Å resolution. We find that scFv binds to a conserved acidic patch on the histone H2A-H2B dimer without perturbing the nucleosome structure. Our results provide an atomic resolution cryo-EM structure of a nucleosome and insight into the structure and function of the CENP-A nucleosome. The scFv approach is applicable to the structural determination of other native-like nucleosomes with distinct DNA sequences. | |||||||||
| 履歴 |
|
- 構造の表示
構造の表示
| ムービー |
 ムービービューア ムービービューア |
|---|---|
| 構造ビューア | EMマップ:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| 添付画像 |
- ダウンロードとリンク
ダウンロードとリンク
-EMDBアーカイブ
| マップデータ |  emd_0586.map.gz emd_0586.map.gz | 59.7 MB |  EMDBマップデータ形式 EMDBマップデータ形式 | |
|---|---|---|---|---|
| ヘッダ (付随情報) |  emd-0586-v30.xml emd-0586-v30.xml emd-0586.xml emd-0586.xml | 18.3 KB 18.3 KB | 表示 表示 |  EMDBヘッダ EMDBヘッダ |
| FSC (解像度算出) |  emd_0586_fsc.xml emd_0586_fsc.xml | 9.2 KB | 表示 |  FSCデータファイル FSCデータファイル |
| 画像 |  emd_0586.png emd_0586.png | 241.9 KB | ||
| Filedesc metadata |  emd-0586.cif.gz emd-0586.cif.gz | 6.8 KB | ||
| アーカイブディレクトリ |  http://ftp.pdbj.org/pub/emdb/structures/EMD-0586 http://ftp.pdbj.org/pub/emdb/structures/EMD-0586 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-0586 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-0586 | HTTPS FTP |
-検証レポート
| 文書・要旨 |  emd_0586_validation.pdf.gz emd_0586_validation.pdf.gz | 552.9 KB | 表示 |  EMDB検証レポート EMDB検証レポート |
|---|---|---|---|---|
| 文書・詳細版 |  emd_0586_full_validation.pdf.gz emd_0586_full_validation.pdf.gz | 552.5 KB | 表示 | |
| XML形式データ |  emd_0586_validation.xml.gz emd_0586_validation.xml.gz | 10.8 KB | 表示 | |
| CIF形式データ |  emd_0586_validation.cif.gz emd_0586_validation.cif.gz | 14.2 KB | 表示 | |
| アーカイブディレクトリ |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-0586 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-0586 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-0586 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-0586 | HTTPS FTP |
-関連構造データ
- リンク
リンク
| EMDBのページ |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| 「今月の分子」の関連する項目 |
- マップ
マップ
| ファイル |  ダウンロード / ファイル: emd_0586.map.gz / 形式: CCP4 / 大きさ: 64 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) ダウンロード / ファイル: emd_0586.map.gz / 形式: CCP4 / 大きさ: 64 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 注釈 | Main EM map after sharpening | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 投影像・断面図 | 画像のコントロール
画像は Spider により作成 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ボクセルのサイズ | X=Y=Z: 0.84875 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 密度 |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 対称性 | 空間群: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 詳細 | EMDB XML:
CCP4マップ ヘッダ情報:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-添付データ
- 試料の構成要素
試料の構成要素
-全体 : CENP-A nucleosome with native alpha satellite DNA sequence
| 全体 | 名称: CENP-A nucleosome with native alpha satellite DNA sequence |
|---|---|
| 要素 |
|
-超分子 #1: CENP-A nucleosome with native alpha satellite DNA sequence
| 超分子 | 名称: CENP-A nucleosome with native alpha satellite DNA sequence タイプ: complex / ID: 1 / 親要素: 0 / 含まれる分子: all |
|---|---|
| 由来(天然) | 生物種:  Homo sapiens (ヒト) Homo sapiens (ヒト) |
-分子 #1: Histone H3-like centromeric protein A
| 分子 | 名称: Histone H3-like centromeric protein A / タイプ: protein_or_peptide / ID: 1 / コピー数: 2 / 光学異性体: LEVO |
|---|---|
| 由来(天然) | 生物種:  Homo sapiens (ヒト) Homo sapiens (ヒト) |
| 分子量 | 理論値: 18.038818 KDa |
| 組換発現 | 生物種:  |
| 配列 | 文字列: MGSSHHHHHH SSGLPRSHMG PRRRSRKPEA PRRRSPSPTP TPGPSRRGPS LGASSHQHSR RRQGWLKEIR KLQKSTHLLI RKLPFSRLA REICVKFTRG VDFNWQAQAL LALQEAAEAF LVHLFEDAYL LTLHAGRVTL FPKDVQLARR IRGLEEGLG UniProtKB: Histone H3-like centromeric protein A |
-分子 #2: Histone H4
| 分子 | 名称: Histone H4 / タイプ: protein_or_peptide / ID: 2 / コピー数: 2 / 光学異性体: LEVO |
|---|---|
| 由来(天然) | 生物種:  Homo sapiens (ヒト) Homo sapiens (ヒト) |
| 分子量 | 理論値: 11.394426 KDa |
| 組換発現 | 生物種:  |
| 配列 | 文字列: MSGRGKGGKG LGKGGAKRHR KVLRDNIQGI TKPAIRRLAR RGGVKRISGL IYEETRGVLK VFLENVIRDA VTYTEHAKRK TVTAMDVVY ALKRQGRTLY GFGG UniProtKB: Histone H4 |
-分子 #3: Histone H2A type 1-B/E
| 分子 | 名称: Histone H2A type 1-B/E / タイプ: protein_or_peptide / ID: 3 / コピー数: 2 / 光学異性体: LEVO |
|---|---|
| 由来(天然) | 生物種:  Homo sapiens (ヒト) Homo sapiens (ヒト) |
| 分子量 | 理論値: 14.165551 KDa |
| 組換発現 | 生物種:  |
| 配列 | 文字列: MSGRGKQGGK ARAKAKTRSS RAGLQFPVGR VHRLLRKGNY SERVGAGAPV YLAAVLEYLT AEILELAGNA ARDNKKTRII PRHLQLAIR NDEELNKLLG RVTIAQGGVL PNIQAVLLPK KTESHHKAKG K UniProtKB: Histone H2A type 1-B/E |
-分子 #4: Histone H2B type 1-J
| 分子 | 名称: Histone H2B type 1-J / タイプ: protein_or_peptide / ID: 4 / コピー数: 2 / 光学異性体: LEVO |
|---|---|
| 由来(天然) | 生物種:  Homo sapiens (ヒト) Homo sapiens (ヒト) |
| 分子量 | 理論値: 13.935239 KDa |
| 組換発現 | 生物種:  |
| 配列 | 文字列: MPEPAKSAPA PKKGSKKAVT KAQKKDGKKR KRSRKESYSI YVYKVLKQVH PDTGISSKAM GIMNSFVNDI FERIAGEASR LAHYNKRST ITSREIQTAV RLLLPGELAK HAVSEGTKAV TKYTSAK UniProtKB: Histone H2B type 1-J |
-分子 #5: DNA (145-MER)
| 分子 | 名称: DNA (145-MER) / タイプ: dna / ID: 5 / コピー数: 1 / 分類: DNA |
|---|---|
| 由来(天然) | 生物種:  Homo sapiens (ヒト) Homo sapiens (ヒト) |
| 分子量 | 理論値: 44.539535 KDa |
| 配列 | 文字列: (DA)(DT)(DC)(DA)(DA)(DT)(DA)(DT)(DC)(DC) (DA)(DC)(DC)(DT)(DG)(DC)(DA)(DG)(DA)(DT) (DT)(DC)(DT)(DA)(DC)(DC)(DA)(DA)(DA) (DA)(DG)(DT)(DG)(DT)(DA)(DT)(DT)(DT)(DG) (DG) (DA)(DA)(DA)(DC)(DT) ...文字列: (DA)(DT)(DC)(DA)(DA)(DT)(DA)(DT)(DC)(DC) (DA)(DC)(DC)(DT)(DG)(DC)(DA)(DG)(DA)(DT) (DT)(DC)(DT)(DA)(DC)(DC)(DA)(DA)(DA) (DA)(DG)(DT)(DG)(DT)(DA)(DT)(DT)(DT)(DG) (DG) (DA)(DA)(DA)(DC)(DT)(DG)(DC)(DT) (DC)(DC)(DA)(DT)(DC)(DA)(DA)(DA)(DA)(DG) (DG)(DC) (DA)(DT)(DG)(DT)(DT)(DC)(DA) (DG)(DC)(DT)(DC)(DT)(DG)(DT)(DG)(DA)(DG) (DT)(DG)(DA) (DA)(DA)(DC)(DT)(DC)(DC) (DA)(DT)(DC)(DA)(DT)(DC)(DA)(DC)(DA)(DA) (DA)(DG)(DA)(DA) (DT)(DA)(DT)(DT)(DC) (DT)(DG)(DA)(DG)(DA)(DA)(DT)(DG)(DC)(DT) (DT)(DC)(DC)(DG)(DT) (DT)(DT)(DG)(DC) (DC)(DT)(DT)(DT)(DT)(DA)(DT)(DA)(DT)(DG) (DA)(DA)(DC)(DT)(DT)(DC) (DC)(DT)(DG) (DA)(DT) |
-分子 #6: DNA (145-MER)
| 分子 | 名称: DNA (145-MER) / タイプ: dna / ID: 6 / コピー数: 1 / 分類: DNA |
|---|---|
| 由来(天然) | 生物種:  Homo sapiens (ヒト) Homo sapiens (ヒト) |
| 分子量 | 理論値: 44.948793 KDa |
| 配列 | 文字列: (DA)(DT)(DC)(DA)(DG)(DG)(DA)(DA)(DG)(DT) (DT)(DC)(DA)(DT)(DA)(DT)(DA)(DA)(DA)(DA) (DG)(DG)(DC)(DA)(DA)(DA)(DC)(DG)(DG) (DA)(DA)(DG)(DC)(DA)(DT)(DT)(DC)(DT)(DC) (DA) (DG)(DA)(DA)(DT)(DA) ...文字列: (DA)(DT)(DC)(DA)(DG)(DG)(DA)(DA)(DG)(DT) (DT)(DC)(DA)(DT)(DA)(DT)(DA)(DA)(DA)(DA) (DG)(DG)(DC)(DA)(DA)(DA)(DC)(DG)(DG) (DA)(DA)(DG)(DC)(DA)(DT)(DT)(DC)(DT)(DC) (DA) (DG)(DA)(DA)(DT)(DA)(DT)(DT)(DC) (DT)(DT)(DT)(DG)(DT)(DG)(DA)(DT)(DG)(DA) (DT)(DG) (DG)(DA)(DG)(DT)(DT)(DT)(DC) (DA)(DC)(DT)(DC)(DA)(DC)(DA)(DG)(DA)(DG) (DC)(DT)(DG) (DA)(DA)(DC)(DA)(DT)(DG) (DC)(DC)(DT)(DT)(DT)(DT)(DG)(DA)(DT)(DG) (DG)(DA)(DG)(DC) (DA)(DG)(DT)(DT)(DT) (DC)(DC)(DA)(DA)(DA)(DT)(DA)(DC)(DA)(DC) (DT)(DT)(DT)(DT)(DG) (DG)(DT)(DA)(DG) (DA)(DA)(DT)(DC)(DT)(DG)(DC)(DA)(DG)(DG) (DT)(DG)(DG)(DA)(DT)(DA) (DT)(DT)(DG) (DA)(DT) |
-実験情報
-構造解析
| 手法 | クライオ電子顕微鏡法 |
|---|---|
 解析 解析 | 単粒子再構成法 |
| 試料の集合状態 | particle |
- 試料調製
試料調製
| 濃度 | 1.5 mg/mL |
|---|---|
| 緩衝液 | pH: 7.4 |
| 凍結 | 凍結剤: ETHANE / チャンバー内湿度: 70 % / 装置: FEI VITROBOT MARK IV / 詳細: blot for 2.5 sec before plunging. |
- 電子顕微鏡法
電子顕微鏡法
| 顕微鏡 | FEI TITAN KRIOS |
|---|---|
| 撮影 | フィルム・検出器のモデル: GATAN K2 SUMMIT (4k x 4k) 検出モード: SUPER-RESOLUTION / デジタル化 - 画像ごとのフレーム数: 2-38 / 撮影したグリッド数: 1 / 実像数: 1256 / 平均露光時間: 15.2 sec. / 平均電子線量: 40.0 e/Å2 詳細: Images were collected in movie-mode at 38 frames over 15.2 seconds |
| 電子線 | 加速電圧: 300 kV / 電子線源:  FIELD EMISSION GUN FIELD EMISSION GUN |
| 電子光学系 | 照射モード: FLOOD BEAM / 撮影モード: BRIGHT FIELD |
| 実験機器 |  モデル: Titan Krios / 画像提供: FEI Company |
 ムービー
ムービー コントローラー
コントローラー

































 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)