5U7V
 
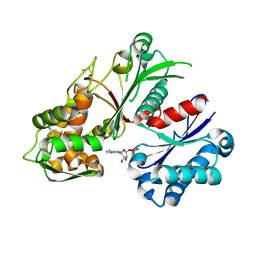 | | Crystal structure of a nucleoside triphosphate diphosphohydrolase (NTPDase) from the legume Trifolium repens in complex with AMP | | 分子名称: | ADENOSINE MONOPHOSPHATE, Apyrase | | 著者 | Cumming, M.H, Summers, E.L, Oulavallickal, T, Roberts, N, Arcus, V.L. | | 登録日 | 2016-12-12 | | 公開日 | 2017-05-31 | | 最終更新日 | 2024-11-20 | | 実験手法 | X-RAY DIFFRACTION (2.15 Å) | | 主引用文献 | Structures and kinetics for plant nucleoside triphosphate diphosphohydrolases support a domain motion catalytic mechanism.
Protein Sci., 26, 2017
|
|
5U7P
 
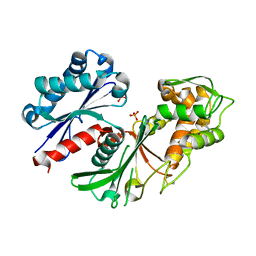 | | Crystal structure of a nucleoside triphosphate diphosphohydrolase (NTPDase) from the legume Trifolium repens | | 分子名称: | Apyrase, PHOSPHATE ION | | 著者 | Cumming, M.H, Summers, E.L, Oulavallickal, T, Roberts, N, Arcus, V.L. | | 登録日 | 2016-12-12 | | 公開日 | 2017-05-31 | | 最終更新日 | 2024-10-16 | | 実験手法 | X-RAY DIFFRACTION (1.89 Å) | | 主引用文献 | Structures and kinetics for plant nucleoside triphosphate diphosphohydrolases support a domain motion catalytic mechanism.
Protein Sci., 26, 2017
|
|
5U7W
 
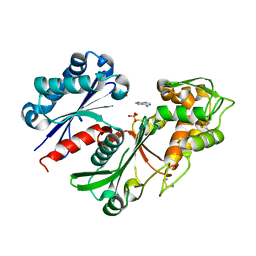 | | Crystal structure of a nucleoside triphosphate diphosphohydrolase (NTPDase) from the legume Trifolium repens in complex with adenine and phosphate | | 分子名称: | ADENINE, Apyrase, PHOSPHATE ION | | 著者 | Cumming, M.H, Summers, E.L, Oulavallickal, T, Roberts, N, Arcus, V.L. | | 登録日 | 2016-12-12 | | 公開日 | 2017-05-31 | | 最終更新日 | 2024-11-06 | | 実験手法 | X-RAY DIFFRACTION (1.76 Å) | | 主引用文献 | Structures and kinetics for plant nucleoside triphosphate diphosphohydrolases support a domain motion catalytic mechanism.
Protein Sci., 26, 2017
|
|
5U7X
 
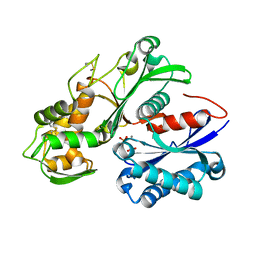 | | Crystal structure of a nucleoside triphosphate diphosphohydrolase (NTPDase) from the legume Vigna unguiculata subsp. cylindrica (Dolichos biflorus) in complex with phosphate and manganese | | 分子名称: | MANGANESE (II) ION, Nod factor binding lectin-nucleotide phosphohydrolase, PHOSPHATE ION | | 著者 | Cumming, M.H, Summers, E.L, Oulavallickal, T, Roberts, N, Arcus, V.L. | | 登録日 | 2016-12-12 | | 公開日 | 2017-05-31 | | 最終更新日 | 2024-11-06 | | 実験手法 | X-RAY DIFFRACTION (2.6 Å) | | 主引用文献 | Structures and kinetics for plant nucleoside triphosphate diphosphohydrolases support a domain motion catalytic mechanism.
Protein Sci., 26, 2017
|
|
