[English] 日本語
 Yorodumi
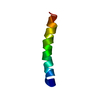
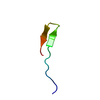
Yorodumi- PDB-1f0g: Cecropin A(1-8)-magainin 2(1-12) L2 in dodecylphosphocholine micelles -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1f0g | ||||||
|---|---|---|---|---|---|---|---|
| Title | Cecropin A(1-8)-magainin 2(1-12) L2 in dodecylphosphocholine micelles | ||||||
 Components Components | CECROPIN A-MAGAININ 2 HYBRID PEPTIDE | ||||||
 Keywords Keywords | ANTIMICROBIAL PROTEIN / Helix-Turn-Helix | ||||||
| Function / homology |  Function and homology information Function and homology informationantimicrobial peptide biosynthetic process / extraorganismal space / regulation of defense response to virus / defense response to fungus / antibacterial humoral response / killing of cells of another organism / defense response to bacterium / defense response to Gram-positive bacterium / innate immune response / extracellular region Similarity search - Function | ||||||
| Method | SOLUTION NMR / Distance Geometry-Dynamical Simulated Annealing Hybrid Method | ||||||
 Authors Authors | Oh, D. / Shin, S.Y. / Lee, S. / Kim, Y. | ||||||
 Citation Citation |  Journal: Biochemistry / Year: 2000 Journal: Biochemistry / Year: 2000Title: Role of the hinge region and the tryptophan residue in the synthetic antimicrobial peptides, cecropin A(1-8)-magainin 2(1-12) and its analogues, on their antibiotic activities and structures. Authors: Oh, D. / Shin, S.Y. / Lee, S. / Kang, J.H. / Kim, S.D. / Ryu, P.D. / Hahm, K.S. / Kim, Y. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1f0g.cif.gz 1f0g.cif.gz | 134.6 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1f0g.ent.gz pdb1f0g.ent.gz | 94.6 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1f0g.json.gz 1f0g.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/f0/1f0g https://data.pdbj.org/pub/pdb/validation_reports/f0/1f0g ftp://data.pdbj.org/pub/pdb/validation_reports/f0/1f0g ftp://data.pdbj.org/pub/pdb/validation_reports/f0/1f0g | HTTPS FTP |
|---|
-Related structure data
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
| |||||||||
| NMR ensembles |
|
- Components
Components
| #1: Protein/peptide | Mass: 2337.998 Da / Num. of mol.: 1 / Mutation: W28L / Source method: obtained synthetically Details: THIS PEPTIDE WAS CHEMICALLY SYNTHESIZED. THE SEQUENCE FOR THIS PEPTIDE IS A HYBRID OF SEQUENCES WHICH OCCUR NATURALLY IN HYALOPHORA CECROPIA (CECROPIA MOTH) AND XENOPUS LAEVIS (AFRICAN CLAWED FROG) References: UniProt: P01507, UniProt: P11006 |
|---|---|
| Has protein modification | Y |
-Experimental details
-Experiment
| Experiment | Method: SOLUTION NMR |
|---|---|
| NMR experiment | Type: 2D NOESY |
- Sample preparation
Sample preparation
| Details | Contents: DODECYLPHOSPHOCHOLINE-d38 MICELLES / Solvent system: 90% H2O/10% D2O |
|---|---|
| Sample conditions | pH: 3.64 / Temperature: 298 K |
| Crystal grow | *PLUS Method: other / Details: NMR |
-NMR measurement
| NMR spectrometer | Type: Bruker AVANCE / Manufacturer: Bruker / Model: AVANCE / Field strength: 400 MHz |
|---|
- Processing
Processing
| NMR software | Name:  X-PLOR / Version: 3.851 / Developer: Brunger, A.T. / Classification: refinement X-PLOR / Version: 3.851 / Developer: Brunger, A.T. / Classification: refinement |
|---|---|
| Refinement | Method: Distance Geometry-Dynamical Simulated Annealing Hybrid Method Software ordinal: 1 |
| NMR ensemble | Conformer selection criteria: structures with the lowest energy Conformers calculated total number: 50 / Conformers submitted total number: 20 |
 Movie
Movie Controller
Controller
















 PDBj
PDBj