[English] 日本語
 Yorodumi
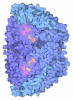
Yorodumi- PDB-9qqt: Methyl-coenzyme M reductase of ANME-2d Candidatus Methanoperedens... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 9qqt | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Methyl-coenzyme M reductase of ANME-2d Candidatus Methanoperedens Vercelli Strain 1 from a bioreactor enrichment culture | ||||||||||||||||||
 Components Components | (Methyl-coenzyme M reductase subunit ...) x 4 | ||||||||||||||||||
 Keywords Keywords | TRANSFERASE / methanotrophy / anaerobic methanotrophic archaea / methane oxidation / methyl-coenzyme M reductase / ANME / MCR / reverse methanogenesis / nickel-dependent enzyme / coenzyme M / coenzyme B / F430 cofactor / post-translational modification | ||||||||||||||||||
| Function / homology |  Function and homology information Function and homology informationcoenzyme-B sulfoethylthiotransferase / coenzyme-B sulfoethylthiotransferase activity / methanogenesis / metal ion binding Similarity search - Function | ||||||||||||||||||
| Biological species |  Candidatus Methanoperedens sp. (archaea) Candidatus Methanoperedens sp. (archaea) | ||||||||||||||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 0.98 Å MOLECULAR REPLACEMENT / Resolution: 0.98 Å | ||||||||||||||||||
 Authors Authors | Mueller, M.-C. / Wagner, T. | ||||||||||||||||||
| Funding support | European Union,  Germany, Germany,  Netherlands, 5items Netherlands, 5items
| ||||||||||||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2025 Journal: Nat Commun / Year: 2025Title: Atomic resolution structures of the methane-activating enzyme in anaerobic methanotrophy reveal extensive post-translational modifications. Authors: Muller, M.C. / Wissink, M. / Mukherjee, P. / Von Possel, N. / Laso-Perez, R. / Engilberge, S. / Carpentier, P. / Kahnt, J. / Wegener, G. / Welte, C.U. / Wagner, T. | ||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  9qqt.cif.gz 9qqt.cif.gz | 1.5 MB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb9qqt.ent.gz pdb9qqt.ent.gz | Display |  PDB format PDB format | |
| PDBx/mmJSON format |  9qqt.json.gz 9qqt.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  9qqt_validation.pdf.gz 9qqt_validation.pdf.gz | 937.1 KB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  9qqt_full_validation.pdf.gz 9qqt_full_validation.pdf.gz | 931.7 KB | Display | |
| Data in XML |  9qqt_validation.xml.gz 9qqt_validation.xml.gz | 140.7 KB | Display | |
| Data in CIF |  9qqt_validation.cif.gz 9qqt_validation.cif.gz | 202.6 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/qq/9qqt https://data.pdbj.org/pub/pdb/validation_reports/qq/9qqt ftp://data.pdbj.org/pub/pdb/validation_reports/qq/9qqt ftp://data.pdbj.org/pub/pdb/validation_reports/qq/9qqt | HTTPS FTP |
-Related structure data
| Related structure data |  9qm5C  9qr1C  9qr3C C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
-Methyl-coenzyme M reductase subunit ... , 4 types, 6 molecules ABECFD
| #1: Protein | Mass: 61873.828 Da / Num. of mol.: 1 / Source method: isolated from a natural source Details: The subunit alpha contains six post-translational modifications: Methylhistidine 272, Methylarginine 286, Hydroxytryptophane 443, Thioglycine 461, Didehydroaspartate 466, and Methylcysteine 468 Source: (natural)  Candidatus Methanoperedens sp. (archaea) / Cell line: / / Organ: / / Plasmid details: / / Variant: / / Strain: Vercelli strain 1 / Tissue: / Candidatus Methanoperedens sp. (archaea) / Cell line: / / Organ: / / Plasmid details: / / Variant: / / Strain: Vercelli strain 1 / Tissue: /References: UniProt: A0A822J3V5, coenzyme-B sulfoethylthiotransferase | ||||
|---|---|---|---|---|---|
| #2: Protein | Mass: 45334.617 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Source: (natural)  Candidatus Methanoperedens sp. (archaea) / Cell line: / / Organ: / / Plasmid details: / / Variant: / / Strain: Vercelli strain 1 / Tissue: / Candidatus Methanoperedens sp. (archaea) / Cell line: / / Organ: / / Plasmid details: / / Variant: / / Strain: Vercelli strain 1 / Tissue: /References: UniProt: A0A822J4Y5, coenzyme-B sulfoethylthiotransferase #3: Protein | Mass: 28253.820 Da / Num. of mol.: 2 / Source method: isolated from a natural source Details: The subunit gamma contains a methylhistidine at position 159 Source: (natural)  Candidatus Methanoperedens sp. (archaea) / Cell line: / / Organ: / / Plasmid details: / / Variant: / / Strain: Vercelli strain 1 / Tissue: / / References: coenzyme-B sulfoethylthiotransferase Candidatus Methanoperedens sp. (archaea) / Cell line: / / Organ: / / Plasmid details: / / Variant: / / Strain: Vercelli strain 1 / Tissue: / / References: coenzyme-B sulfoethylthiotransferase#4: Protein | | Mass: 61857.828 Da / Num. of mol.: 1 / Source method: isolated from a natural source Details: The subunit alpha contains six post-translational modifications: Methylhistidine 272, Methylarginine 286, Hydroxytryptophane 443, Thioglycine 461, Didehydroaspartate 466, and Methylcysteine 468 Source: (natural)  Candidatus Methanoperedens sp. (archaea) / Cell line: / / Organ: / / Plasmid details: / / Variant: / / Strain: Vercelli strain 1 / Tissue: / Candidatus Methanoperedens sp. (archaea) / Cell line: / / Organ: / / Plasmid details: / / Variant: / / Strain: Vercelli strain 1 / Tissue: /References: UniProt: A0A822J3V5, coenzyme-B sulfoethylthiotransferase |
-Non-polymers , 7 types, 3324 molecules 




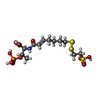







| #5: Chemical | ChemComp-K / | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| #6: Chemical | | #7: Chemical | ChemComp-EDO / #8: Chemical | #9: Chemical | #10: Chemical | #11: Water | ChemComp-HOH / | |
-Details
| Has ligand of interest | Y |
|---|---|
| Has protein modification | Y |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.19 Å3/Da / Density % sol: 43.88 % / Description: Thick yellow brick-shaped |
|---|---|
| Crystal grow | Temperature: 291.15 K / Method: vapor diffusion, sitting drop / pH: 6.9 Details: Crystals were produced on a Junior Clover plate using a crystallization solution containing 20 % w/v polyethylene glycol 3,350 and 200 mM sodium thiocyanate pH 6.9. The reservoir contained ...Details: Crystals were produced on a Junior Clover plate using a crystallization solution containing 20 % w/v polyethylene glycol 3,350 and 200 mM sodium thiocyanate pH 6.9. The reservoir contained 100 ul crystallization solution and drops of 2 ul solution were mixed with 3 ul protein at a concentration of 40 mg/ml in 25 mM Tris/HCl pH 8.0, 10% v/v glycerol, 100 mM NaCl and 2 mM dithiothreitol. Crystals were soaked in the crystallization solution containing 20 % v/v ethylene glycol before freezing in liquid nitrogen. Temp details: / |
-Data collection
| Diffraction | Mean temperature: 100 K / Serial crystal experiment: N |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  ESRF ESRF  / Beamline: BM07 / Wavelength: 0.9793 Å / Beamline: BM07 / Wavelength: 0.9793 Å |
| Detector | Type: DECTRIS PILATUS 6M / Detector: PIXEL / Date: Feb 15, 2024 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.9793 Å / Relative weight: 1 |
| Reflection | Resolution: 0.977→94.911 Å / Num. obs: 996827 / % possible obs: 83.9 % / Redundancy: 7.6 % / CC1/2: 0.998 / Rmerge(I) obs: 0.079 / Rpim(I) all: 0.03 / Rrim(I) all: 0.085 / Net I/σ(I): 13 |
| Reflection shell | Resolution: 0.977→1.035 Å / Redundancy: 7 % / Rmerge(I) obs: 0.8 / Mean I/σ(I) obs: 1.9 / Num. unique obs: 49841 / CC1/2: 0.754 / Rpim(I) all: 0.32 / Rrim(I) all: 0.863 / % possible all: 62.6 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT / Resolution: 0.98→41.71 Å / SU ML: 0.08 / Cross valid method: FREE R-VALUE / σ(F): 1.34 / Phase error: 16.7 / Stereochemistry target values: ML MOLECULAR REPLACEMENT / Resolution: 0.98→41.71 Å / SU ML: 0.08 / Cross valid method: FREE R-VALUE / σ(F): 1.34 / Phase error: 16.7 / Stereochemistry target values: ML
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.1 Å / Solvent model: FLAT BULK SOLVENT MODEL | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 0.98→41.71 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell |
|
 Movie
Movie Controller
Controller


 PDBj
PDBj