[English] 日本語
 Yorodumi
Yorodumi- PDB-9h90: Cryo-EM structure of the Vibrio natrigens 30S ribosomal subunit i... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 9h90 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of the Vibrio natrigens 30S ribosomal subunit in complex with spectinomycin. | |||||||||
 Components Components |
| |||||||||
 Keywords Keywords | RIBOSOME / Vibrio natriegens / Bac5 / Bactenecin 5 / 50S / V. natriegens | |||||||||
| Function / homology |  Function and homology information Function and homology informationribosome biogenesis / ribosomal small subunit biogenesis / small ribosomal subunit / small ribosomal subunit rRNA binding / cytosolic small ribosomal subunit / tRNA binding / rRNA binding / structural constituent of ribosome / ribosome / translation ...ribosome biogenesis / ribosomal small subunit biogenesis / small ribosomal subunit / small ribosomal subunit rRNA binding / cytosolic small ribosomal subunit / tRNA binding / rRNA binding / structural constituent of ribosome / ribosome / translation / ribonucleoprotein complex / mRNA binding / RNA binding / cytoplasm / cytosol Similarity search - Function | |||||||||
| Biological species |  Vibrio natriegens (bacteria) Vibrio natriegens (bacteria) | |||||||||
| Method | ELECTRON MICROSCOPY / single particle reconstruction / cryo EM / Resolution: 2.8 Å | |||||||||
 Authors Authors | Raulf, K.F. / Koller, T.O. / Beckert, B. / Morici, M. / Lepak, A. / Bange, G. / Wilson, D.N. | |||||||||
| Funding support |  Germany, 2items Germany, 2items
| |||||||||
 Citation Citation |  Journal: Nucleic Acids Res / Year: 2025 Journal: Nucleic Acids Res / Year: 2025Title: The structure of the Vibrio natriegens 70S ribosome in complex with the proline-rich antimicrobial peptide Bac5(1-17). Authors: Karoline Raulf / Timm O Koller / Bertrand Beckert / Alexander Lepak / Martino Morici / Mario Mardirossian / Marco Scocchi / Gert Bange / Daniel N Wilson /   Abstract: Proline-rich antimicrobial peptides (PrAMPs) are produced as part of the innate immune response of animals, insects, and plants. The well-characterized mammalian PrAMP bactenecin-5 (Bac5) has been ...Proline-rich antimicrobial peptides (PrAMPs) are produced as part of the innate immune response of animals, insects, and plants. The well-characterized mammalian PrAMP bactenecin-5 (Bac5) has been shown to help fight bacterial infection by binding to the bacterial ribosome and inhibiting protein synthesis. In the absence of Bac5-ribosome structures, the binding mode of Bac5 and exact mechanism of action has remained unclear. Here, we present a cryo-electron microscopy structure of Bac5 in complex with the 70S ribosome from the Gram-negative marine bacterium Vibrio natriegens. The structure shows that, despite sequence similarity to Bac7 and other type I PrAMPs, Bac5 displays a completely distinct mode of interaction with the ribosomal exit tunnel. Bac5 overlaps with the binding site of both A- and P-site transfer RNAs bound at the peptidyltransferase center, suggesting that this type I PrAMP can interfere with late stages of translation initiation as well as early stages of elongation. Collectively, our study presents a ribosome structure from V. natriegens, a fast-growing bacterium that has interesting biotechnological and synthetic biology applications, as well as providing additional insights into the diverse binding modes that type I PrAMPs can utilize to inhibit protein synthesis. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  9h90.cif.gz 9h90.cif.gz | 1.2 MB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb9h90.ent.gz pdb9h90.ent.gz | 937 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  9h90.json.gz 9h90.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/h9/9h90 https://data.pdbj.org/pub/pdb/validation_reports/h9/9h90 ftp://data.pdbj.org/pub/pdb/validation_reports/h9/9h90 ftp://data.pdbj.org/pub/pdb/validation_reports/h9/9h90 | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  51946MC  9h91C M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
-30S ribosomal protein ... , 16 types, 16 molecules uCohfedItrqplkDJ
| #1: Protein | Mass: 8512.055 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  Vibrio natriegens (bacteria) / References: UniProt: A0AAN1CUP2 Vibrio natriegens (bacteria) / References: UniProt: A0AAN1CUP2 |
|---|---|
| #2: Protein | Mass: 25598.854 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  Vibrio natriegens (bacteria) / References: UniProt: A0AAN0Y138 Vibrio natriegens (bacteria) / References: UniProt: A0AAN0Y138 |
| #4: Protein | Mass: 10084.664 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  Vibrio natriegens (bacteria) / References: UniProt: A0AAN0Y3D6 Vibrio natriegens (bacteria) / References: UniProt: A0AAN0Y3D6 |
| #5: Protein | Mass: 14027.628 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  Vibrio natriegens (bacteria) / References: UniProt: A0AAN0Y0S4 Vibrio natriegens (bacteria) / References: UniProt: A0AAN0Y0S4 |
| #6: Protein | Mass: 14994.074 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  Vibrio natriegens (bacteria) / References: UniProt: A0AAN0Y4F0 Vibrio natriegens (bacteria) / References: UniProt: A0AAN0Y4F0 |
| #7: Protein | Mass: 17609.348 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  Vibrio natriegens (bacteria) / References: UniProt: A0AAN1CUS4 Vibrio natriegens (bacteria) / References: UniProt: A0AAN1CUS4 |
| #8: Protein | Mass: 23392.094 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  Vibrio natriegens (bacteria) / References: UniProt: A0AAN0XZY8 Vibrio natriegens (bacteria) / References: UniProt: A0AAN0XZY8 |
| #9: Protein | Mass: 14638.934 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  Vibrio natriegens (bacteria) / References: UniProt: A0AAN0Y103 Vibrio natriegens (bacteria) / References: UniProt: A0AAN0Y103 |
| #11: Protein | Mass: 9590.353 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  Vibrio natriegens (bacteria) / References: UniProt: A0AAN1CUQ1 Vibrio natriegens (bacteria) / References: UniProt: A0AAN1CUQ1 |
| #12: Protein | Mass: 8861.315 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  Vibrio natriegens (bacteria) / References: UniProt: A0AAN0Y440 Vibrio natriegens (bacteria) / References: UniProt: A0AAN0Y440 |
| #13: Protein | Mass: 9605.193 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  Vibrio natriegens (bacteria) / References: UniProt: A0AAN0XZY2 Vibrio natriegens (bacteria) / References: UniProt: A0AAN0XZY2 |
| #14: Protein | Mass: 9080.427 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  Vibrio natriegens (bacteria) / References: UniProt: A0AAN0Y460 Vibrio natriegens (bacteria) / References: UniProt: A0AAN0Y460 |
| #15: Protein | Mass: 13738.043 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  Vibrio natriegens (bacteria) / References: UniProt: A0AAN0Y4G9 Vibrio natriegens (bacteria) / References: UniProt: A0AAN0Y4G9 |
| #16: Protein | Mass: 13916.035 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  Vibrio natriegens (bacteria) / References: UniProt: A0AAN0Y004 Vibrio natriegens (bacteria) / References: UniProt: A0AAN0Y004 |
| #17: Protein | Mass: 11481.414 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  Vibrio natriegens (bacteria) / References: UniProt: A0AAN0Y0L1 Vibrio natriegens (bacteria) / References: UniProt: A0AAN0Y0L1 |
| #18: Protein | Mass: 11741.673 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  Vibrio natriegens (bacteria) / References: UniProt: A0AAN0Y0R4 Vibrio natriegens (bacteria) / References: UniProt: A0AAN0Y0R4 |
-Protein / RNA chain , 2 types, 2 molecules Ga
| #10: RNA chain | Mass: 500566.594 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  Vibrio natriegens (bacteria) Vibrio natriegens (bacteria) |
|---|---|
| #3: Protein | Mass: 17765.768 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  Vibrio natriegens (bacteria) / References: UniProt: A0AAN0Y3T9 Vibrio natriegens (bacteria) / References: UniProt: A0AAN0Y3T9 |
-Non-polymers , 2 types, 5 molecules 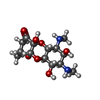


| #19: Chemical | ChemComp-SCM / |
|---|---|
| #20: Water | ChemComp-HOH / |
-Details
| Has ligand of interest | Y |
|---|---|
| Has protein modification | N |
-Experimental details
-Experiment
| Experiment | Method: ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method: single particle reconstruction |
- Sample preparation
Sample preparation
| Component | Name: Structure of the Vibrio natriegens 30S ribosomal subunit in complex with spectinomycin Type: RIBOSOME / Entity ID: #1-#18 / Source: NATURAL | ||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular weight | Experimental value: NO | ||||||||||||||||||||||||||||||||||||||||
| Source (natural) | Organism:  Vibrio natriegens (bacteria) Vibrio natriegens (bacteria) | ||||||||||||||||||||||||||||||||||||||||
| Buffer solution | pH: 7.5 | ||||||||||||||||||||||||||||||||||||||||
| Buffer component |
| ||||||||||||||||||||||||||||||||||||||||
| Specimen | Embedding applied: NO / Shadowing applied: NO / Staining applied: NO / Vitrification applied: YES / Details: 8 OD/ml ribosome concentration | ||||||||||||||||||||||||||||||||||||||||
| Vitrification | Cryogen name: ETHANE-PROPANE / Humidity: 100 % / Chamber temperature: 277 K |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: TFS KRIOS |
| Electron gun | Electron source:  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD / Nominal defocus max: 1200 nm / Nominal defocus min: 700 nm |
| Image recording | Electron dose: 1 e/Å2 / Detector mode: COUNTING / Film or detector model: FEI FALCON II (4k x 4k) |
- Processing
Processing
| EM software | Name: REFMAC / Version: 5.8.0425 / Category: model refinement | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CTF correction | Type: PHASE FLIPPING AND AMPLITUDE CORRECTION | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3D reconstruction | Resolution: 2.8 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 294068 / Symmetry type: POINT | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic model building | Protocol: RIGID BODY FIT | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement | Resolution: 2.8→233.42 Å / Cor.coef. Fo:Fc: 0.75 / SU B: 13.523 / SU ML: 0.238 / ESU R: 0.254 Stereochemistry target values: MAXIMUM LIKELIHOOD WITH PHASES Details: HYDROGENS HAVE BEEN USED IF PRESENT IN THE INPUT
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Solvent model: PARAMETERS FOR MASK CACLULATION | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 64.992 Å2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: 1 / Total: 47492 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller



 PDBj
PDBj





























