+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 9dw9 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Phosphorylated (E1371Q)CFTR in complex with PKA-C | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Components Components |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Keywords Keywords | HYDROLASE / CFTR / PKA / complex | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Function / homology |  Function and homology information Function and homology informationpositive regulation of voltage-gated chloride channel activity / : / Sec61 translocon complex binding / channel-conductance-controlling ATPase / intracellularly ATP-gated chloride channel activity / CD209 (DC-SIGN) signaling / HDL assembly / Regulation of insulin secretion / positive regulation of enamel mineralization / Rap1 signalling ...positive regulation of voltage-gated chloride channel activity / : / Sec61 translocon complex binding / channel-conductance-controlling ATPase / intracellularly ATP-gated chloride channel activity / CD209 (DC-SIGN) signaling / HDL assembly / Regulation of insulin secretion / positive regulation of enamel mineralization / Rap1 signalling / Ion homeostasis / transepithelial water transport / RHO GTPases regulate CFTR trafficking / PKA activation in glucagon signalling / DARPP-32 events / CREB1 phosphorylation through the activation of Adenylate Cyclase / GPER1 signaling / Factors involved in megakaryocyte development and platelet production / Loss of Nlp from mitotic centrosomes / Recruitment of mitotic centrosome proteins and complexes / Loss of proteins required for interphase microtubule organization from the centrosome / Anchoring of the basal body to the plasma membrane / RET signaling / AURKA Activation by TPX2 / amelogenesis / Interleukin-3, Interleukin-5 and GM-CSF signaling / intracellular pH elevation / Recruitment of NuMA to mitotic centrosomes / VEGFA-VEGFR2 Pathway / PKA activation / MAPK6/MAPK4 signaling / GLI3 is processed to GLI3R by the proteasome / chloride channel inhibitor activity / : / Regulation of PLK1 Activity at G2/M Transition / Hedgehog 'off' state / multicellular organismal-level water homeostasis / Golgi-associated vesicle membrane / cholesterol transport / bicarbonate transport / bicarbonate transmembrane transporter activity / chloride channel regulator activity / membrane hyperpolarization / vesicle docking involved in exocytosis / chloride transmembrane transporter activity / cAMP-dependent protein kinase / regulation of protein processing / cAMP-dependent protein kinase activity / protein localization to lipid droplet / regulation of bicellular tight junction assembly / cAMP-dependent protein kinase complex / cellular response to parathyroid hormone stimulus / Mitochondrial protein degradation / cholesterol biosynthetic process / Glucagon-like Peptide-1 (GLP1) regulates insulin secretion / regulation of osteoblast differentiation / cellular response to cold / High laminar flow shear stress activates signaling by PIEZO1 and PECAM1:CDH5:KDR in endothelial cells / Vasopressin regulates renal water homeostasis via Aquaporins / sperm capacitation / negative regulation of glycolytic process through fructose-6-phosphate / ciliary base / RHOQ GTPase cycle / chloride channel activity / protein kinase A regulatory subunit binding / intracellular potassium ion homeostasis / mesoderm formation / positive regulation of exocytosis / plasma membrane raft / ATPase-coupled transmembrane transporter activity / axoneme / positive regulation of insulin secretion involved in cellular response to glucose stimulus / ABC-type transporter activity / chloride channel complex / sperm flagellum / postsynaptic modulation of chemical synaptic transmission / 14-3-3 protein binding / regulation of proteasomal protein catabolic process / negative regulation of TORC1 signaling / protein serine/threonine/tyrosine kinase activity / cellular response to glucagon stimulus / positive regulation of gluconeogenesis / acrosomal vesicle / cellular response to forskolin / chloride transmembrane transport / protein export from nucleus / positive regulation of phagocytosis / cellular response to cAMP / response to endoplasmic reticulum stress / positive regulation of protein export from nucleus / PDZ domain binding / negative regulation of smoothened signaling pathway / neural tube closure / neuromuscular junction / clathrin-coated endocytic vesicle membrane / cellular response to glucose stimulus / establishment of localization in cell / Defective CFTR causes cystic fibrosis / positive regulation of cholesterol biosynthetic process / Late endosomal microautophagy Similarity search - Function | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Method | ELECTRON MICROSCOPY / single particle reconstruction / cryo EM / Resolution: 2.8 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Authors Authors | Fiedorczuk, K. / Chen, J. / Csanady, L. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Funding support |  United States, 2items United States, 2items
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Citation Citation |  Journal: Proc Natl Acad Sci U S A / Year: 2024 Journal: Proc Natl Acad Sci U S A / Year: 2024Title: The structures of protein kinase A in complex with CFTR: Mechanisms of phosphorylation and noncatalytic activation. Authors: Karol Fiedorczuk / Iordan Iordanov / Csaba Mihályi / Andras Szollosi / László Csanády / Jue Chen /   Abstract: Protein kinase A (PKA) is a key regulator of cellular functions by selectively phosphorylating numerous substrates, including ion channels, enzymes, and transcription factors. It has long served as a ...Protein kinase A (PKA) is a key regulator of cellular functions by selectively phosphorylating numerous substrates, including ion channels, enzymes, and transcription factors. It has long served as a model system for understanding the eukaryotic kinases. Using cryoelectron microscopy, we present complex structures of the PKA catalytic subunit (PKA-C) bound to a full-length protein substrate, the cystic fibrosis transmembrane conductance regulator (CFTR)-an ion channel vital to human health. CFTR gating requires phosphorylation of its regulatory (R) domain. Unphosphorylated CFTR engages PKA-C at two locations, establishing two "catalytic stations" near to, but not directly involving, the R domain. This configuration, coupled with the conformational flexibility of the R domain, permits transient interactions of the eleven spatially separated phosphorylation sites. Furthermore, we determined two structures of the open-pore CFTR stabilized by PKA-C, providing a molecular basis to understand how PKA-C stimulates CFTR currents even in the absence of phosphorylation. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  9dw9.cif.gz 9dw9.cif.gz | 325.5 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb9dw9.ent.gz pdb9dw9.ent.gz | 251.6 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  9dw9.json.gz 9dw9.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  9dw9_validation.pdf.gz 9dw9_validation.pdf.gz | 1.8 MB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  9dw9_full_validation.pdf.gz 9dw9_full_validation.pdf.gz | 1.8 MB | Display | |
| Data in XML |  9dw9_validation.xml.gz 9dw9_validation.xml.gz | 59.2 KB | Display | |
| Data in CIF |  9dw9_validation.cif.gz 9dw9_validation.cif.gz | 88.6 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/dw/9dw9 https://data.pdbj.org/pub/pdb/validation_reports/dw/9dw9 ftp://data.pdbj.org/pub/pdb/validation_reports/dw/9dw9 ftp://data.pdbj.org/pub/pdb/validation_reports/dw/9dw9 | HTTPS FTP |
-Related structure data
| Related structure data |  47239MC  9dw4C  9dw5C  9dw7C  9dw8C M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
-Protein , 2 types, 2 molecules AG
| #1: Protein | Mass: 168414.453 Da / Num. of mol.: 1 / Mutation: E1371Q Source method: isolated from a genetically manipulated source Details: phosphorylated (E1371Q)CFTR / Source: (gene. exp.)  Homo sapiens (human) / Gene: CFTR, ABCC7 / Production host: Homo sapiens (human) / Gene: CFTR, ABCC7 / Production host:  Homo sapiens (human) Homo sapiens (human)References: UniProt: P13569, channel-conductance-controlling ATPase |
|---|---|
| #2: Protein | Mass: 40757.633 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Details: PKA-C / Source: (gene. exp.)   |
-Non-polymers , 7 types, 22 molecules 




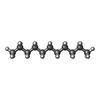







| #3: Chemical | ChemComp-CLR / | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| #4: Chemical | ChemComp-D10 / #5: Chemical | ChemComp-MG / #6: Chemical | #7: Chemical | ChemComp-CL / #8: Chemical | #9: Water | ChemComp-HOH / | |
-Details
| Has ligand of interest | Y |
|---|---|
| Has protein modification | Y |
-Experimental details
-Experiment
| Experiment | Method: ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method: single particle reconstruction |
- Sample preparation
Sample preparation
| Component | Name: Phosphorylated (E1371Q)CFTR in complex with PKA-C / Type: COMPLEX / Entity ID: #1-#2 / Source: MULTIPLE SOURCES |
|---|---|
| Molecular weight | Value: 0.21 MDa / Experimental value: NO |
| Source (natural) | Organism:  |
| Source (recombinant) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Buffer solution | pH: 7.4 |
| Specimen | Conc.: 5 mg/ml / Embedding applied: NO / Shadowing applied: NO / Staining applied: NO / Vitrification applied: YES |
| Specimen support | Grid material: COPPER / Grid type: Quantifoil R0.6/1 |
| Vitrification | Instrument: FEI VITROBOT MARK IV / Cryogen name: ETHANE / Humidity: 100 % |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: TFS KRIOS |
| Electron gun | Electron source:  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD / Nominal defocus max: 1800 nm / Nominal defocus min: 800 nm |
| Image recording | Electron dose: 52.5 e/Å2 / Film or detector model: GATAN K3 (6k x 4k) |
- Processing
Processing
| EM software | Name: PHENIX / Category: model refinement | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CTF correction | Type: PHASE FLIPPING AND AMPLITUDE CORRECTION | ||||||||||||||||||||||||
| 3D reconstruction | Resolution: 2.8 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 47950 / Symmetry type: POINT | ||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller







 PDBj
PDBj



















