[English] 日本語
 Yorodumi
Yorodumi- PDB-8vr4: Structure of Mycobacterium smegmatis 50S ribosomal subunit bound ... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 8vr4 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of Mycobacterium smegmatis 50S ribosomal subunit bound to HflX and erythromycin:50S-HflX-A-Ery | ||||||||||||
 Components Components |
| ||||||||||||
 Keywords Keywords | RIBOSOME / ribosome splitting / Mycobacterium smegmatis 50S / HflX / disordered 23S rRNA helices / erythromycin / translation | ||||||||||||
| Function / homology |  Function and homology information Function and homology informationlarge ribosomal subunit / transferase activity / ribosome binding / ribosomal large subunit assembly / 5S rRNA binding / large ribosomal subunit rRNA binding / cytosolic large ribosomal subunit / cytoplasmic translation / tRNA binding / negative regulation of translation ...large ribosomal subunit / transferase activity / ribosome binding / ribosomal large subunit assembly / 5S rRNA binding / large ribosomal subunit rRNA binding / cytosolic large ribosomal subunit / cytoplasmic translation / tRNA binding / negative regulation of translation / rRNA binding / structural constituent of ribosome / ribosome / translation / ribonucleoprotein complex / GTPase activity / mRNA binding / GTP binding / metal ion binding / cytoplasm Similarity search - Function | ||||||||||||
| Biological species |  Mycolicibacterium smegmatis MC2 155 (bacteria) Mycolicibacterium smegmatis MC2 155 (bacteria) | ||||||||||||
| Method | ELECTRON MICROSCOPY / single particle reconstruction / cryo EM / Resolution: 2.8 Å | ||||||||||||
 Authors Authors | Majumdar, S. / Koripella, R.K. / Sharma, M.R. / Manjari, S.R. / Banavali, N.K. / Agrawal, R.K. | ||||||||||||
| Funding support |  United States, 3items United States, 3items
| ||||||||||||
 Citation Citation |  Journal: Proc Natl Acad Sci U S A / Year: 2025 Journal: Proc Natl Acad Sci U S A / Year: 2025Title: HflX-mediated drug resistance through ribosome splitting and rRNA disordering in mycobacteria. Authors: Soneya Majumdar / Amuliya Kashyap / Ravi K Koripella / Manjuli R Sharma / Kelley Hurst-Hess / Swati R Manjari / Nilesh K Banavali / Pallavi Ghosh / Rajendra K Agrawal /  Abstract: HflX is a highly conserved ribosome-associated GTPase implicated in rescuing stalled ribosomes and mediating antibiotic resistance in several bacteria, including macrolide-lincosamide antibiotic ...HflX is a highly conserved ribosome-associated GTPase implicated in rescuing stalled ribosomes and mediating antibiotic resistance in several bacteria, including macrolide-lincosamide antibiotic resistance in mycobacteria. Mycobacterial HflXs carry a distinct N-terminal extension (NTE) and a small insertion, as compared to their eubacterial homologs. Here, we present several high-resolution cryo-EM structures of mycobacterial HflX in complex with the 70S ribosome and its 50S subunit, with and without antibiotics. These structures reveal a distinct mechanism for HflX-mediated ribosome splitting and antibiotic resistance in mycobacteria. Our findings indicate that the NTE of mycobacterial HflX induces persistent disordering of multiple 23S rRNA helices, facilitating the dissociation of the 70S ribosome and generating an inactive pool of 50S subunits. During this process, HflX undergoes a large conformational change that stabilizes its NTE. Mycobacterial HflX also acts as an anti-association factor by binding to predissociated 50S subunits. Our structures show that a mycobacteria-specific insertion in HflX reaches far into the peptidyl transferase center (PTC), such that it would overlap with the ribosome-bound macrolide antibiotics. However, in the presence of antibiotics, this insertion retracts, adjusts around, and interacts with the antibiotic molecules. These results suggest that mycobacterial HflX is agnostic to antibiotic presence in the PTC. It mediates antibiotic resistance by splitting antibiotic-stalled 70S ribosomes and inactivating the resulting 50S subunits. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  8vr4.cif.gz 8vr4.cif.gz | 2.1 MB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb8vr4.ent.gz pdb8vr4.ent.gz | 1.7 MB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  8vr4.json.gz 8vr4.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/vr/8vr4 https://data.pdbj.org/pub/pdb/validation_reports/vr/8vr4 ftp://data.pdbj.org/pub/pdb/validation_reports/vr/8vr4 ftp://data.pdbj.org/pub/pdb/validation_reports/vr/8vr4 | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  43476MC  8vioC  8vk0C  8vk7C  8vkiC  8vkwC  8vpkC  8vr8C  8vrlC C: citing same article ( M: map data used to model this data |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
+50S Ribosomal Protein ... , 30 types, 30 molecules 23CDEFGHJKLMOPQRSTUVWXYZbcdefg
-Protein , 2 types, 2 molecules 4N
| #3: Protein | Mass: 50637.094 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Mycolicibacterium smegmatis MC2 155 (bacteria) Mycolicibacterium smegmatis MC2 155 (bacteria)Gene: hflX, MSMEG_2736 Production host:  References: UniProt: A0QVY1 |
|---|---|
| #15: Protein | Mass: 15774.240 Da / Num. of mol.: 1 / Source method: isolated from a natural source Source: (natural)  Mycolicibacterium smegmatis MC2 155 (bacteria) Mycolicibacterium smegmatis MC2 155 (bacteria)References: UniProt: A0QSD8 |
-RNA chain , 2 types, 2 molecules BA
| #4: RNA chain | Mass: 38061.816 Da / Num. of mol.: 1 / Source method: isolated from a natural source Source: (natural)  Mycolicibacterium smegmatis MC2 155 (bacteria) Mycolicibacterium smegmatis MC2 155 (bacteria)References: GenBank: 118168627 |
|---|---|
| #34: RNA chain | Mass: 1012140.938 Da / Num. of mol.: 1 / Source method: isolated from a natural source Source: (natural)  Mycolicibacterium smegmatis MC2 155 (bacteria) Mycolicibacterium smegmatis MC2 155 (bacteria)References: GenBank: 118168627 |
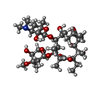
-Non-polymers , 2 types, 2 molecules 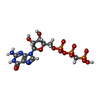

| #35: Chemical | ChemComp-GCP / |
|---|---|
| #36: Chemical | ChemComp-ERY / |
-Details
| Has ligand of interest | Y |
|---|---|
| Has protein modification | N |
-Experimental details
-Experiment
| Experiment | Method: ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method: single particle reconstruction |
- Sample preparation
Sample preparation
| Component | Name: Mycobacterium smegmatis 50S ribosome bound to HflX-GMPPCP complex and Erythromycin Type: RIBOSOME / Entity ID: #1-#34 / Source: NATURAL | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular weight | Experimental value: NO | |||||||||||||||||||||||||
| Source (natural) | Organism:  Mycolicibacterium smegmatis MC2 155 (bacteria) Mycolicibacterium smegmatis MC2 155 (bacteria) | |||||||||||||||||||||||||
| Buffer solution | pH: 7.5 Details: 20 mM HEPES-K at pH 7.5, 30 mM ammonium chloride, 10 mM magnesium chloride, and 5 mM beta-mercaptoethanol | |||||||||||||||||||||||||
| Buffer component |
| |||||||||||||||||||||||||
| Specimen | Embedding applied: NO / Shadowing applied: NO / Staining applied: NO / Vitrification applied: YES | |||||||||||||||||||||||||
| Specimen support | Grid material: COPPER / Grid mesh size: 300 divisions/in. / Grid type: Quantifoil R1.2/1.3 | |||||||||||||||||||||||||
| Vitrification | Instrument: FEI VITROBOT MARK IV / Cryogen name: ETHANE / Humidity: 100 % / Chamber temperature: 277.15 K |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: FEI TITAN KRIOS |
| Electron gun | Electron source:  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD / Nominal magnification: 81000 X / Nominal defocus max: 1600 nm / Nominal defocus min: 1000 nm / Cs: 0.001 mm |
| Specimen holder | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER |
| Image recording | Electron dose: 62.87 e/Å2 / Detector mode: COUNTING / Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Num. of grids imaged: 1 / Num. of real images: 12145 |
| Image scans | Movie frames/image: 40 / Used frames/image: 1-40 |
- Processing
Processing
| EM software | Name: PHENIX / Version: 1.20.1_4487: / Category: model refinement | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CTF correction | Type: PHASE FLIPPING AND AMPLITUDE CORRECTION | ||||||||||||||||||||||||
| Particle selection | Num. of particles selected: 1747436 | ||||||||||||||||||||||||
| Symmetry | Point symmetry: C1 (asymmetric) | ||||||||||||||||||||||||
| 3D reconstruction | Resolution: 2.8 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 46709 / Symmetry type: POINT | ||||||||||||||||||||||||
| Atomic model building | B value: 31.1 / Protocol: FLEXIBLE FIT / Space: REAL / Target criteria: Cross-correlation coefficient | ||||||||||||||||||||||||
| Atomic model building |
| ||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller













 PDBj
PDBj


































