+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 8pvb | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of GABAAR determined by cryoEM at 100 keV | |||||||||||||||||||||
 Components Components |
| |||||||||||||||||||||
 Keywords Keywords | MEMBRANE PROTEIN / Pentameric ligand-gated ion channel / Neurotrasmitter receptor / GABA(A) receptor | |||||||||||||||||||||
| Function / homology |  Function and homology information Function and homology informationcircadian sleep/wake cycle, REM sleep / reproductive behavior / hard palate development / cellular response to histamine / GABA receptor activation / inner ear receptor cell development / inhibitory synapse assembly / GABA-A receptor activity / GABA-gated chloride ion channel activity / GABA-A receptor complex ...circadian sleep/wake cycle, REM sleep / reproductive behavior / hard palate development / cellular response to histamine / GABA receptor activation / inner ear receptor cell development / inhibitory synapse assembly / GABA-A receptor activity / GABA-gated chloride ion channel activity / GABA-A receptor complex / innervation / response to anesthetic / postsynaptic specialization membrane / inhibitory postsynaptic potential / gamma-aminobutyric acid signaling pathway / synaptic transmission, GABAergic / cellular response to zinc ion / exploration behavior / motor behavior / roof of mouth development / Signaling by ERBB4 / cochlea development / social behavior / chloride channel complex / cytoplasmic vesicle membrane / chloride transmembrane transport / cerebellum development / learning / transmitter-gated monoatomic ion channel activity involved in regulation of postsynaptic membrane potential / GABA-ergic synapse / memory / dendritic spine / postsynaptic membrane / response to xenobiotic stimulus / cell surface / signal transduction / identical protein binding / plasma membrane Similarity search - Function | |||||||||||||||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||||||||||||||
| Method | ELECTRON MICROSCOPY / single particle reconstruction / cryo EM / Resolution: 3.6 Å | |||||||||||||||||||||
 Authors Authors | McMullan, G. / Naydenova, K. / Mihaylov, D. / Peet, M.J. / Wilson, H. / Yamashita, K. / Dickerson, J.L. / Chen, S. / Cannone, G. / Lee, Y. ...McMullan, G. / Naydenova, K. / Mihaylov, D. / Peet, M.J. / Wilson, H. / Yamashita, K. / Dickerson, J.L. / Chen, S. / Cannone, G. / Lee, Y. / Hutchings, K.A. / Gittins, O. / Sobhy, M. / Wells, T. / El-Gomati, M.M. / Dalby, J. / Meffert, M. / Schulze-Briese, C. / Henderson, R. / Russo, C.J. | |||||||||||||||||||||
| Funding support |  United Kingdom, 6items United Kingdom, 6items
| |||||||||||||||||||||
 Citation Citation |  Journal: Proc Natl Acad Sci U S A / Year: 2023 Journal: Proc Natl Acad Sci U S A / Year: 2023Title: Structure determination by cryoEM at 100 keV. Authors: Greg McMullan / Katerina Naydenova / Daniel Mihaylov / Keitaro Yamashita / Mathew J Peet / Hugh Wilson / Joshua L Dickerson / Shaoxia Chen / Giuseppe Cannone / Yang Lee / Katherine A ...Authors: Greg McMullan / Katerina Naydenova / Daniel Mihaylov / Keitaro Yamashita / Mathew J Peet / Hugh Wilson / Joshua L Dickerson / Shaoxia Chen / Giuseppe Cannone / Yang Lee / Katherine A Hutchings / Olivia Gittins / Mohamed A Sobhy / Torquil Wells / Mohamed M El-Gomati / Jason Dalby / Matthias Meffert / Clemens Schulze-Briese / Richard Henderson / Christopher J Russo /    Abstract: Electron cryomicroscopy can, in principle, determine the structures of most biological molecules but is currently limited by access, specimen preparation difficulties, and cost. We describe a purpose- ...Electron cryomicroscopy can, in principle, determine the structures of most biological molecules but is currently limited by access, specimen preparation difficulties, and cost. We describe a purpose-built instrument operating at 100 keV-including advances in electron optics, detection, and processing-that makes structure determination fast and simple at a fraction of current costs. The instrument attains its theoretical performance limits, allowing atomic resolution imaging of gold test specimens and biological molecular structure determination in hours. We demonstrate its capabilities by determining the structures of eleven different specimens, ranging in size from 140 kDa to 2 MDa, using a fraction of the data normally required. CryoEM with a microscope designed specifically for high-efficiency, on-the-spot imaging of biological molecules will expand structural biology to a wide range of previously intractable problems. | |||||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  8pvb.cif.gz 8pvb.cif.gz | 148.8 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb8pvb.ent.gz pdb8pvb.ent.gz | 85 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  8pvb.json.gz 8pvb.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  8pvb_validation.pdf.gz 8pvb_validation.pdf.gz | 1.4 MB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  8pvb_full_validation.pdf.gz 8pvb_full_validation.pdf.gz | 1.4 MB | Display | |
| Data in XML |  8pvb_validation.xml.gz 8pvb_validation.xml.gz | 32.7 KB | Display | |
| Data in CIF |  8pvb_validation.cif.gz 8pvb_validation.cif.gz | 46.2 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/pv/8pvb https://data.pdbj.org/pub/pdb/validation_reports/pv/8pvb ftp://data.pdbj.org/pub/pdb/validation_reports/pv/8pvb ftp://data.pdbj.org/pub/pdb/validation_reports/pv/8pvb | HTTPS FTP |
-Related structure data
| Related structure data |  17960MC  8pv9C  8pvaC  8pvcC  8pvdC  8pveC  8pvfC  8pvgC  8pvhC  8pviC  8pvjC M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | x 5
| ||||||||||||||||||||||||
| Noncrystallographic symmetry (NCS) | NCS oper:
|
- Components
Components
-Protein / Antibody , 2 types, 2 molecules AO
| #1: Protein | Mass: 45744.285 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: GABRB3 / Production host: Homo sapiens (human) / Gene: GABRB3 / Production host:  Homo sapiens (human) / References: UniProt: P28472 Homo sapiens (human) / References: UniProt: P28472 |
|---|---|
| #2: Antibody | Mass: 56300.629 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   |
-Sugars , 2 types, 2 molecules
| #3: Polysaccharide | 2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose Source method: isolated from a genetically manipulated source |
|---|---|
| #4: Polysaccharide | alpha-D-mannopyranose-(1-3)-[alpha-D-mannopyranose-(1-6)]beta-D-mannopyranose-(1-4)-2-acetamido-2- ...alpha-D-mannopyranose-(1-3)-[alpha-D-mannopyranose-(1-6)]beta-D-mannopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose Source method: isolated from a genetically manipulated source |
-Non-polymers , 5 types, 9 molecules 

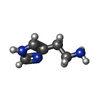

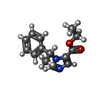




| #5: Chemical | ChemComp-R16 / | ||||||
|---|---|---|---|---|---|---|---|
| #6: Chemical | ChemComp-D10 / #7: Chemical | ChemComp-HSM / | #8: Chemical | #9: Chemical | ChemComp-V8D / | |
-Details
| Has ligand of interest | N |
|---|---|
| Has protein modification | Y |
-Experimental details
-Experiment
| Experiment | Method: ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method: single particle reconstruction |
- Sample preparation
Sample preparation
| Component | Name: Human GABA(A)R-beta3 homopentamer in complex with Megabody-25 in lipid nanodisc Type: COMPLEX / Entity ID: #1-#2 / Source: RECOMBINANT |
|---|---|
| Molecular weight | Units: MEGADALTONS / Experimental value: NO |
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Source (recombinant) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Buffer solution | pH: 7.4 |
| Specimen | Embedding applied: NO / Shadowing applied: NO / Staining applied: NO / Vitrification applied: YES |
| Specimen support | Grid material: GOLD / Grid type: UltrAuFoil R0./1 |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy imaging
Electron microscopy imaging
| Microscopy | Model: JEOL 1400/HR + YPS FEG |
|---|---|
| Electron gun | Electron source:  FIELD EMISSION GUN / Accelerating voltage: 100 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 100 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD / Nominal defocus max: 2000 nm / Nominal defocus min: 500 nm |
| Specimen holder | Cryogen: NITROGEN Specimen holder model: GATAN 626 SINGLE TILT LIQUID NITROGEN CRYO TRANSFER HOLDER |
| Image recording | Electron dose: 51 e/Å2 / Film or detector model: DECTRIS SINGLA (1k x 1k) |
| Image scans | Sampling size: 75 µm / Width: 1030 / Height: 1066 |
- Processing
Processing
| EM software | Name: Servalcat / Version: 0.4.27 / Category: model refinement |
|---|---|
| CTF correction | Type: PHASE FLIPPING AND AMPLITUDE CORRECTION |
| Symmetry | Point symmetry: C5 (5 fold cyclic) |
| 3D reconstruction | Resolution: 3.6 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 23477 / Symmetry type: POINT |
| Atomic model building | Space: RECIPROCAL |
| Atomic model building | PDB-ID: 7a5v Accession code: 7a5v / Source name: PDB / Type: experimental model |
 Movie
Movie Controller
Controller













 PDBj
PDBj










