+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 8g9v | ||||||
|---|---|---|---|---|---|---|---|
| Title | Crystal structures of 17-beta-hydroxysteroid dehydrogenase 13 | ||||||
 Components Components | 17-beta-hydroxysteroid dehydrogenase 13 | ||||||
 Keywords Keywords | OXIDOREDUCTASE/INHIBITOR / 17 beta-hydroxysteroid dehydrogenase 13 / lipid droplet associated protein / inhibitor / OXIDOREDUCTASE / OXIDOREDUCTASE-INHIBITOR complex | ||||||
| Function / homology |  Function and homology information Function and homology informationLipid particle organization / all-trans-retinol dehydrogenase (NAD+) / steroid dehydrogenase activity / 17beta-estradiol 17-dehydrogenase / estradiol 17-beta-dehydrogenase [NAD(P)+] activity / all-trans-retinol dehydrogenase (NAD+) activity / Oxidoreductases; Acting on the CH-OH group of donors; With NAD+ or NADP+ as acceptor / oxidoreductase activity, acting on the CH-OH group of donors, NAD or NADP as acceptor / positive regulation of lipid biosynthetic process / lipid droplet ...Lipid particle organization / all-trans-retinol dehydrogenase (NAD+) / steroid dehydrogenase activity / 17beta-estradiol 17-dehydrogenase / estradiol 17-beta-dehydrogenase [NAD(P)+] activity / all-trans-retinol dehydrogenase (NAD+) activity / Oxidoreductases; Acting on the CH-OH group of donors; With NAD+ or NADP+ as acceptor / oxidoreductase activity, acting on the CH-OH group of donors, NAD or NADP as acceptor / positive regulation of lipid biosynthetic process / lipid droplet / lipid metabolic process / endoplasmic reticulum / cytosol Similarity search - Function | ||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.645 Å MOLECULAR REPLACEMENT / Resolution: 2.645 Å | ||||||
 Authors Authors | Liu, S. | ||||||
| Funding support | 1items
| ||||||
 Citation Citation |  Journal: Nat Commun / Year: 2023 Journal: Nat Commun / Year: 2023Title: Structural basis of lipid-droplet localization of 17-beta-hydroxysteroid dehydrogenase 13. Authors: Liu, S. / Sommese, R.F. / Nedoma, N.L. / Stevens, L.M. / Dutra, J.K. / Zhang, L. / Edmonds, D.J. / Wang, Y. / Garnsey, M. / Clasquin, M.F. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  8g9v.cif.gz 8g9v.cif.gz | 890.7 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb8g9v.ent.gz pdb8g9v.ent.gz | 746.7 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  8g9v.json.gz 8g9v.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  8g9v_validation.pdf.gz 8g9v_validation.pdf.gz | 4.9 MB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  8g9v_full_validation.pdf.gz 8g9v_full_validation.pdf.gz | 5 MB | Display | |
| Data in XML |  8g9v_validation.xml.gz 8g9v_validation.xml.gz | 81.5 KB | Display | |
| Data in CIF |  8g9v_validation.cif.gz 8g9v_validation.cif.gz | 105.5 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/g9/8g9v https://data.pdbj.org/pub/pdb/validation_reports/g9/8g9v ftp://data.pdbj.org/pub/pdb/validation_reports/g9/8g9v ftp://data.pdbj.org/pub/pdb/validation_reports/g9/8g9v | HTTPS FTP |
-Related structure data
| Related structure data |  8g84C  8g89C  8g93C C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| 2 | 
| ||||||||
| 3 | 
| ||||||||
| 4 | 
| ||||||||
| Unit cell |
|
- Components
Components
-Protein , 1 types, 8 molecules ABCDEFGH
| #1: Protein | Mass: 35502.348 Da / Num. of mol.: 8 / Mutation: Q60K, I62R, R71H, E161K Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: HSD17B13, SCDR9, SDR16C3, HMFN0376, UNQ497/PRO1014 / Cell line (production host): SF9 / Production host: Homo sapiens (human) / Gene: HSD17B13, SCDR9, SDR16C3, HMFN0376, UNQ497/PRO1014 / Cell line (production host): SF9 / Production host:  References: UniProt: Q7Z5P4, Oxidoreductases; Acting on the CH-OH group of donors |
|---|
-Non-polymers , 5 types, 60 molecules 
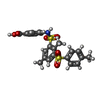







| #2: Chemical | ChemComp-NAD / #3: Chemical | ChemComp-YYC / #4: Chemical | ChemComp-CE1 / #5: Chemical | ChemComp-SO4 / #6: Water | ChemComp-HOH / | |
|---|
-Details
| Has ligand of interest | Y |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.75 Å3/Da / Density % sol: 55.19 % |
|---|---|
| Crystal grow | Temperature: 293 K / Method: vapor diffusion, sitting drop / Details: 25% PEG3350, 0.1 M Bis-Tris pH 5.5, 0.2M Li2SO4 |
-Data collection
| Diffraction | Mean temperature: 100 K / Serial crystal experiment: N |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  APS APS  / Beamline: 17-ID / Wavelength: 1 Å / Beamline: 17-ID / Wavelength: 1 Å |
| Detector | Type: DECTRIS EIGER X 16M / Detector: PIXEL / Date: Mar 12, 2020 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1 Å / Relative weight: 1 |
| Reflection | Resolution: 2.645→90.95 Å / Num. obs: 67940 / % possible obs: 93.4 % / Redundancy: 3.4 % / CC1/2: 0.99 / Rpim(I) all: 0.083 / Net I/σ(I): 7.9 |
| Reflection shell | Resolution: 2.645→2.897 Å / Mean I/σ(I) obs: 1.5 / Num. unique obs: 3398 / CC1/2: 0.462 / Rpim(I) all: 0.589 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT / Resolution: 2.645→37.91 Å / Cor.coef. Fo:Fc: 0.928 / Cor.coef. Fo:Fc free: 0.907 / Cross valid method: THROUGHOUT / σ(F): 0 / SU Rfree Blow DPI: 0.349 MOLECULAR REPLACEMENT / Resolution: 2.645→37.91 Å / Cor.coef. Fo:Fc: 0.928 / Cor.coef. Fo:Fc free: 0.907 / Cross valid method: THROUGHOUT / σ(F): 0 / SU Rfree Blow DPI: 0.349 Details: HYDROGENS WERE FULLY REFINED WITH ZERO OCCUPANCY AT NUCLEAR POSITION.
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 61.47 Å2
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine analyze | Luzzati coordinate error obs: 0.38 Å | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.645→37.91 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 2.65→2.8 Å / Total num. of bins used: 51
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Refine-ID: X-RAY DIFFRACTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group |
|
 Movie
Movie Controller
Controller



 PDBj
PDBj







