[English] 日本語
 Yorodumi
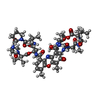
Yorodumi- PDB-7x97: Crystal structure of actinomycin D-echinomycin-d(AGCCCGT/ACGGGCT)... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 7x97 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Crystal structure of actinomycin D-echinomycin-d(AGCCCGT/ACGGGCT) complex | ||||||||||||
 Components Components |
| ||||||||||||
 Keywords Keywords | DNA/ANTIBIOTIC / C:G Watson-Crick base pair / Single-strand DNA twisting / Actinomycin D / Echinomycin / DNA-antibiotic complex / DNA | ||||||||||||
| Function / homology | Actinomycin D / Echinomycin / : / 2-CARBOXYQUINOXALINE / DNA Function and homology information Function and homology information | ||||||||||||
| Biological species |  Streptomyces (bacteria) Streptomyces (bacteria)synthetic construct (others) | ||||||||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.95 Å MOLECULAR REPLACEMENT / Resolution: 1.95 Å | ||||||||||||
 Authors Authors | Kao, S.H. / Satange, R.B. / Hou, M.H. | ||||||||||||
| Funding support |  Taiwan, 2items Taiwan, 2items
| ||||||||||||
 Citation Citation |  Journal: Nucleic Acids Res. / Year: 2022 Journal: Nucleic Acids Res. / Year: 2022Title: Staggered intercalation of DNA duplexes with base-pair modulation by two distinct drug molecules induces asymmetric backbone twisting and structure polymorphism. Authors: Satange, R. / Kao, S.H. / Chien, C.M. / Chou, S.H. / Lin, C.C. / Neidle, S. / Hou, M.H. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  7x97.cif.gz 7x97.cif.gz | 39.5 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb7x97.ent.gz pdb7x97.ent.gz | 26 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  7x97.json.gz 7x97.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  7x97_validation.pdf.gz 7x97_validation.pdf.gz | 455.9 KB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  7x97_full_validation.pdf.gz 7x97_full_validation.pdf.gz | 458.6 KB | Display | |
| Data in XML |  7x97_validation.xml.gz 7x97_validation.xml.gz | 5.2 KB | Display | |
| Data in CIF |  7x97_validation.cif.gz 7x97_validation.cif.gz | 6.1 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/x9/7x97 https://data.pdbj.org/pub/pdb/validation_reports/x9/7x97 ftp://data.pdbj.org/pub/pdb/validation_reports/x9/7x97 ftp://data.pdbj.org/pub/pdb/validation_reports/x9/7x97 | HTTPS FTP |
-Related structure data
| Related structure data |  7x6rC  7x9fC  7xdjC  7dq0S S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
| |||||||||||||||||||||
| Unit cell |
| |||||||||||||||||||||
| Components on special symmetry positions |
|
- Components
Components
-DNA chain , 2 types, 2 molecules AB
| #1: DNA chain | Mass: 2098.399 Da / Num. of mol.: 1 / Source method: obtained synthetically / Source: (synth.) synthetic construct (others) |
|---|---|
| #2: DNA chain | Mass: 2138.423 Da / Num. of mol.: 1 / Source method: obtained synthetically / Source: (synth.) synthetic construct (others) |
-Protein/peptide , 2 types, 2 molecules GC
-Non-polymers , 5 types, 36 molecules 


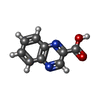





| #5: Chemical | ChemComp-ZN / #6: Chemical | ChemComp-K / | #7: Chemical | #8: Chemical | #9: Water | ChemComp-HOH / | |
|---|
-Details
| Compound details | ACTINOMYCIN D IS A BICYCLIC PEPTIDE, A MEMBER OF THE ACTINOMYCIN FAMILY. HERE, ACTINOMYCIN D IS ...ACTINOMYCI |
|---|---|
| Has ligand of interest | N |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.93 Å3/Da / Density % sol: 58.03 % |
|---|---|
| Crystal grow | Temperature: 293 K / Method: vapor diffusion, sitting drop / pH: 6 Details: 0.25 mM Oligonucleotides, 0.25 mM Echinomycin, 0.125 mM Actinomycin D, 2.5 mM Sodium cacodylate (pH 6.5), 0.5 mM Spermine tetrahydrochloride, 6 mM Zinc chloride, 3.5 mM Potassium chloride, ...Details: 0.25 mM Oligonucleotides, 0.25 mM Echinomycin, 0.125 mM Actinomycin D, 2.5 mM Sodium cacodylate (pH 6.5), 0.5 mM Spermine tetrahydrochloride, 6 mM Zinc chloride, 3.5 mM Potassium chloride, PEG200 3% (v/v), Reservoir PEG 200 (30% v/v) |
-Data collection
| Diffraction | Mean temperature: 100 K / Serial crystal experiment: N |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  NSRRC NSRRC  / Beamline: TPS 05A / Wavelength: 0.99984 Å / Beamline: TPS 05A / Wavelength: 0.99984 Å |
| Detector | Type: RAYONIX MX300-HS / Detector: CCD / Date: Mar 15, 2019 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.99984 Å / Relative weight: 1 |
| Reflection | Resolution: 1.95→30 Å / Num. obs: 7161 / % possible obs: 97.8 % / Redundancy: 12.3 % / Biso Wilson estimate: 19.74 Å2 / Rmerge(I) obs: 0.057 / Rpim(I) all: 0.018 / Rrim(I) all: 0.06 / Χ2: 0.888 / Net I/σ(I): 7.7 / Num. measured all: 76141 |
| Reflection shell | Resolution: 1.95→2.02 Å / Redundancy: 7.8 % / Rmerge(I) obs: 0.399 / Num. unique obs: 702 / CC1/2: 0.999 / Rpim(I) all: 0.01 / Rrim(I) all: 0.034 / Χ2: 0.859 / % possible all: 93.1 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 7DQ0 Resolution: 1.95→25.57 Å / SU ML: 0.26 / Cross valid method: THROUGHOUT / σ(F): 1.36 / Phase error: 28.61 / Stereochemistry target values: ML
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å / Solvent model: FLAT BULK SOLVENT MODEL | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso max: 116.56 Å2 / Biso mean: 25.47 Å2 / Biso min: 7.01 Å2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: final / Resolution: 1.95→25.57 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Refine-ID: X-RAY DIFFRACTION / Rfactor Rfree error: 0 / Total num. of bins used: 5
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Refine-ID: X-RAY DIFFRACTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group |
|
 Movie
Movie Controller
Controller


 PDBj
PDBj