+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 7s0z | ||||||
|---|---|---|---|---|---|---|---|
| Title | Structures of TcdB in complex with R-Ras | ||||||
 Components Components |
| ||||||
 Keywords Keywords | Hydrolase/Transferase / toxin / substrate / enzyme / Hydrolase-Transferase complex | ||||||
| Function / homology |  Function and homology information Function and homology informationleukocyte differentiation / positive regulation of endothelial cell-matrix adhesion via fibronectin / negative regulation of Schwann cell migration / Schwann cell migration / regulation of phosphatidylinositol 3-kinase/protein kinase B signal transduction / positive regulation of vasculogenesis / SEMA3A-Plexin repulsion signaling by inhibiting Integrin adhesion / Sema4D mediated inhibition of cell attachment and migration / face morphogenesis / host cell cytosol ...leukocyte differentiation / positive regulation of endothelial cell-matrix adhesion via fibronectin / negative regulation of Schwann cell migration / Schwann cell migration / regulation of phosphatidylinositol 3-kinase/protein kinase B signal transduction / positive regulation of vasculogenesis / SEMA3A-Plexin repulsion signaling by inhibiting Integrin adhesion / Sema4D mediated inhibition of cell attachment and migration / face morphogenesis / host cell cytosol / glycosyltransferase activity / regulation of ERK1 and ERK2 cascade / positive regulation of endothelial cell migration / cysteine-type peptidase activity / host cell endosome membrane / small monomeric GTPase / positive regulation of angiogenesis / GDP binding / toxin activity / Ras protein signal transduction / focal adhesion / GTPase activity / lipid binding / GTP binding / protein-containing complex binding / host cell plasma membrane / proteolysis / extracellular exosome / extracellular region / metal ion binding / membrane / plasma membrane Similarity search - Function | ||||||
| Biological species |  Clostridioides difficile (bacteria) Clostridioides difficile (bacteria) Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.34 Å MOLECULAR REPLACEMENT / Resolution: 2.34 Å | ||||||
 Authors Authors | Zheng, L. / Rongsheng, J. / Peng, C. | ||||||
| Funding support |  United States, 1items United States, 1items
| ||||||
 Citation Citation |  Journal: Sci Adv / Year: 2021 Journal: Sci Adv / Year: 2021Title: Structural basis for selective modification of Rho and Ras GTPases by Clostridioides difficile toxin B. Authors: Liu, Z. / Zhang, S. / Chen, P. / Tian, S. / Zeng, J. / Perry, K. / Dong, M. / Jin, R. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  7s0z.cif.gz 7s0z.cif.gz | 317 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb7s0z.ent.gz pdb7s0z.ent.gz | 252.6 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  7s0z.json.gz 7s0z.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  7s0z_validation.pdf.gz 7s0z_validation.pdf.gz | 1.8 MB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  7s0z_full_validation.pdf.gz 7s0z_full_validation.pdf.gz | 1.8 MB | Display | |
| Data in XML |  7s0z_validation.xml.gz 7s0z_validation.xml.gz | 58.5 KB | Display | |
| Data in CIF |  7s0z_validation.cif.gz 7s0z_validation.cif.gz | 80.8 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/s0/7s0z https://data.pdbj.org/pub/pdb/validation_reports/s0/7s0z ftp://data.pdbj.org/pub/pdb/validation_reports/s0/7s0z ftp://data.pdbj.org/pub/pdb/validation_reports/s0/7s0z | HTTPS FTP |
-Related structure data
| Related structure data |  7s0yC  2fn4S  6oq7S S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| 2 | 
| ||||||||
| Unit cell |
|
- Components
Components
-Protein , 2 types, 4 molecules BACD
| #1: Protein | Mass: 62642.113 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Clostridioides difficile (bacteria) / Gene: tcdB, SAMEA708418_03270 / Production host: Clostridioides difficile (bacteria) / Gene: tcdB, SAMEA708418_03270 / Production host:  References: UniProt: M4NKV9, Transferases; Glycosyltransferases; Hexosyltransferases #2: Protein | Mass: 19945.336 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: RRAS / Production host: Homo sapiens (human) / Gene: RRAS / Production host:  References: UniProt: P10301, Hydrolases; Acting on acid anhydrides; Acting on GTP to facilitate cellular and subcellular movement |
|---|
-Sugars , 1 types, 2 molecules 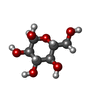
| #9: Sugar |
|---|
-Non-polymers , 9 types, 588 molecules 
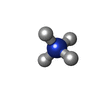















| #3: Chemical | | #4: Chemical | ChemComp-NH4 / #5: Chemical | ChemComp-EDO / #6: Chemical | ChemComp-ACT / #7: Chemical | ChemComp-PEG / | #8: Chemical | #10: Chemical | ChemComp-MG / #11: Chemical | #12: Water | ChemComp-HOH / | |
|---|
-Details
| Has ligand of interest | Y |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.62 Å3/Da / Density % sol: 52.99 % |
|---|---|
| Crystal grow | Temperature: 291 K / Method: vapor diffusion, hanging drop Details: 0.2M ammonium acetate, 0.1M sodium citrate tribasic dihydrate, pH 4.8, and 17% PEG 4000 |
-Data collection
| Diffraction | Mean temperature: 100 K / Serial crystal experiment: N |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  APS APS  / Beamline: 24-ID-C / Wavelength: 0.97918 Å / Beamline: 24-ID-C / Wavelength: 0.97918 Å |
| Detector | Type: DECTRIS EIGER2 X 16M / Detector: PIXEL / Date: Dec 11, 2020 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.97918 Å / Relative weight: 1 |
| Reflection | Resolution: 2.34→112.11 Å / Num. obs: 70923 / % possible obs: 98.9 % / Redundancy: 3.4 % / CC1/2: 0.993 / Rmerge(I) obs: 0.085 / Net I/σ(I): 9.6 |
| Reflection shell | Resolution: 2.34→2.39 Å / Num. unique obs: 4599 / CC1/2: 0.693 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 6OQ7,2FN4 Resolution: 2.34→76.02 Å / Cor.coef. Fo:Fc: 0.948 / Cor.coef. Fo:Fc free: 0.922 / SU B: 8.499 / SU ML: 0.197 / Cross valid method: THROUGHOUT / ESU R: 0.403 / ESU R Free: 0.248 / Stereochemistry target values: MAXIMUM LIKELIHOOD / Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.2 Å / Solvent model: MASK | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 37.61 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.34→76.02 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller












 PDBj
PDBj

















