[English] 日本語
 Yorodumi
Yorodumi- PDB-5jha: Structure of Phosphoinositide 3-kinase gamma (PI3K) bound to the ... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 5jha | ||||||
|---|---|---|---|---|---|---|---|
| Title | Structure of Phosphoinositide 3-kinase gamma (PI3K) bound to the potent inhibitor PIKin2 | ||||||
 Components Components | Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform | ||||||
 Keywords Keywords | TRANSFERASE / Inhibitor / Lipid kinase / PI3K | ||||||
| Function / homology |  Function and homology information Function and homology informationnegative regulation of cardiac muscle contraction / negative regulation of triglyceride catabolic process / natural killer cell chemotaxis / secretory granule localization / neutrophil extravasation / phosphatidylinositol-4-phosphate 3-kinase / positive regulation of acute inflammatory response / respiratory burst involved in defense response / T cell chemotaxis / negative regulation of fibroblast apoptotic process ...negative regulation of cardiac muscle contraction / negative regulation of triglyceride catabolic process / natural killer cell chemotaxis / secretory granule localization / neutrophil extravasation / phosphatidylinositol-4-phosphate 3-kinase / positive regulation of acute inflammatory response / respiratory burst involved in defense response / T cell chemotaxis / negative regulation of fibroblast apoptotic process / phosphatidylinositol 3-kinase complex, class IB / regulation of calcium ion transmembrane transport / 1-phosphatidylinositol-4-phosphate 3-kinase activity / Co-stimulation by ICOS / sphingosine-1-phosphate receptor signaling pathway / phosphatidylinositol 3-kinase complex, class IA / phosphatidylinositol-3-phosphate biosynthetic process / 1-phosphatidylinositol-4,5-bisphosphate 3-kinase activity / phosphatidylinositol-4,5-bisphosphate 3-kinase / phosphatidylinositol 3-kinase / dendritic cell chemotaxis / 1-phosphatidylinositol-3-kinase activity / Erythropoietin activates Phosphoinositide-3-kinase (PI3K) / mast cell degranulation / phosphatidylinositol-mediated signaling / hepatocyte apoptotic process / regulation of cell adhesion mediated by integrin / phosphatidylinositol phosphate biosynthetic process / Synthesis of PIPs at the plasma membrane / positive regulation of MAP kinase activity / positive regulation of Rac protein signal transduction / CD28 dependent PI3K/Akt signaling / regulation of angiogenesis / T cell proliferation / ephrin receptor binding / GPVI-mediated activation cascade / neutrophil chemotaxis / positive regulation of endothelial cell migration / cellular response to cAMP / T cell activation / positive regulation of cytokine production / phosphatidylinositol 3-kinase/protein kinase B signal transduction / platelet aggregation / endocytosis / Constitutive Signaling by Aberrant PI3K in Cancer / G beta:gamma signalling through PI3Kgamma / cell migration / PIP3 activates AKT signaling / positive regulation of cytosolic calcium ion concentration / PI5P, PP2A and IER3 Regulate PI3K/AKT Signaling / angiogenesis / phospholipase C-activating G protein-coupled receptor signaling pathway / adaptive immune response / positive regulation of phosphatidylinositol 3-kinase/protein kinase B signal transduction / protein kinase activity / non-specific serine/threonine protein kinase / immune response / G protein-coupled receptor signaling pathway / inflammatory response / innate immune response / protein serine kinase activity / protein serine/threonine kinase activity / ATP binding / identical protein binding / membrane / plasma membrane / cytosol / cytoplasm Similarity search - Function | ||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.51 Å MOLECULAR REPLACEMENT / Resolution: 2.51 Å | ||||||
 Authors Authors | Burke, J.E. / Inglis, A.J. / Williams, R.L. | ||||||
| Funding support |  United Kingdom, 1items United Kingdom, 1items
| ||||||
 Citation Citation |  Journal: Nat Commun / Year: 2017 Journal: Nat Commun / Year: 2017Title: Deconvolution of Buparlisib's mechanism of action defines specific PI3K and tubulin inhibitors for therapeutic intervention. Authors: Bohnacker, T. / Prota, A.E. / Beaufils, F. / Burke, J.E. / Melone, A. / Inglis, A.J. / Rageot, D. / Sele, A.M. / Cmiljanovic, V. / Cmiljanovic, N. / Bargsten, K. / Aher, A. / Akhmanova, A. / ...Authors: Bohnacker, T. / Prota, A.E. / Beaufils, F. / Burke, J.E. / Melone, A. / Inglis, A.J. / Rageot, D. / Sele, A.M. / Cmiljanovic, V. / Cmiljanovic, N. / Bargsten, K. / Aher, A. / Akhmanova, A. / Diaz, J.F. / Fabbro, D. / Zvelebil, M. / Williams, R.L. / Steinmetz, M.O. / Wymann, M.P. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  5jha.cif.gz 5jha.cif.gz | 354.8 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb5jha.ent.gz pdb5jha.ent.gz | 287.3 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  5jha.json.gz 5jha.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/jh/5jha https://data.pdbj.org/pub/pdb/validation_reports/jh/5jha ftp://data.pdbj.org/pub/pdb/validation_reports/jh/5jha ftp://data.pdbj.org/pub/pdb/validation_reports/jh/5jha | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  5jhbC  5m7eC  5m7gC  5m8dC  5m8gC  3sd5S S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 109898.203 Da / Num. of mol.: 1 / Mutation: Fragment: Residues 144-1102 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: PIK3CG / Production host: Homo sapiens (human) / Gene: PIK3CG / Production host:  References: UniProt: P48736, phosphatidylinositol-4,5-bisphosphate 3-kinase, non-specific serine/threonine protein kinase | ||
|---|---|---|---|
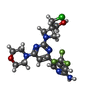
| #2: Chemical | ChemComp-6K7 / [ | ||
| #3: Chemical | ChemComp-SO4 / #4: Water | ChemComp-HOH / | |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.38 Å3/Da / Density % sol: 48.27 % |
|---|---|
| Crystal grow | Temperature: 293 K / Method: vapor diffusion, sitting drop / pH: 7.5 Details: 17% PEG 4000, 250 mM (NH4)2SO4, and 100mM Tris pH-7.5 |
-Data collection
| Diffraction | Mean temperature: 100 K | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  Diamond Diamond  / Beamline: I04 / Wavelength: 0.9793 Å / Beamline: I04 / Wavelength: 0.9793 Å | |||||||||||||||
| Detector | Type: DECTRIS PILATUS 6M-F / Detector: PIXEL / Date: Sep 21, 2013 | |||||||||||||||
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray | |||||||||||||||
| Radiation wavelength | Wavelength: 0.9793 Å / Relative weight: 1 | |||||||||||||||
| Reflection | Resolution: 2.51→61.51 Å / Num. obs: 34660 / % possible obs: 97.8 % / Redundancy: 3.4 % / Biso Wilson estimate: 73.53 Å2 / CC1/2: 0.998 / Rmerge(I) obs: 0.059 / Rpim(I) all: 0.038 / Rrim(I) all: 0.07 / Net I/σ(I): 17.7 / Num. measured all: 116517 | |||||||||||||||
| Reflection shell |
|
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 3sd5 Resolution: 2.51→61.51 Å / SU ML: 0.38 / Cross valid method: FREE R-VALUE / σ(F): 1.34 / Phase error: 29.11
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso max: 228.55 Å2 / Biso mean: 100.9621 Å2 / Biso min: 42.25 Å2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: final / Resolution: 2.51→61.51 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Refine-ID: X-RAY DIFFRACTION / Total num. of bins used: 12
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Refine-ID: X-RAY DIFFRACTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group |
|
 Movie
Movie Controller
Controller












 PDBj
PDBj