| Entry | Database: PDB / ID: 7run
|
|---|
| Title | Crystal structure of phosphorylated RET tyrosine kinase domain complexed with a pyrrolo[2,3-d]pyrimidine inhibitor. |
|---|
 Components Components | Proto-oncogene tyrosine-protein kinase receptor Ret |
|---|
 Keywords Keywords | TRANSFERASE/TRANSFERASE INHIBITOR / tyrosine-protein kinase / inhibitor / cell adhesion / TRANSFERASE-TRANSFERASE INHIBITOR complex |
|---|
| Function / homology |  Function and homology information Function and homology information
Peyer's patch morphogenesis / GDF15-GFRAL signaling pathway / positive regulation of metanephric glomerulus development / ureter maturation / embryonic epithelial tube formation / glial cell-derived neurotrophic factor receptor signaling pathway / lymphocyte migration into lymphoid organs / posterior midgut development / Formation of the ureteric bud / membrane protein proteolysis ...Peyer's patch morphogenesis / GDF15-GFRAL signaling pathway / positive regulation of metanephric glomerulus development / ureter maturation / embryonic epithelial tube formation / glial cell-derived neurotrophic factor receptor signaling pathway / lymphocyte migration into lymphoid organs / posterior midgut development / Formation of the ureteric bud / membrane protein proteolysis / Formation of the nephric duct / enteric nervous system development / neuron cell-cell adhesion / plasma membrane protein complex / neuron maturation / positive regulation of extrinsic apoptotic signaling pathway in absence of ligand / positive regulation of cell adhesion mediated by integrin / neural crest cell migration / ureteric bud development / response to pain / regulation of axonogenesis / homophilic cell-cell adhesion / RET signaling / positive regulation of cell size / regulation of cell adhesion / cellular response to retinoic acid / NPAS4 regulates expression of target genes / transmembrane receptor protein tyrosine kinase activity / axon guidance / cell surface receptor protein tyrosine kinase signaling pathway / positive regulation of neuron projection development / receptor protein-tyrosine kinase / MAPK cascade / signaling receptor activity / RAF/MAP kinase cascade / protein tyrosine kinase activity / positive regulation of phosphatidylinositol 3-kinase/protein kinase B signal transduction / receptor complex / endosome membrane / positive regulation of MAPK cascade / positive regulation of cell migration / axon / calcium ion binding / positive regulation of gene expression / positive regulation of DNA-templated transcription / signal transduction / ATP binding / plasma membraneSimilarity search - Function Tyrosine-protein kinase, Ret receptor / Tyrosine-protein kinase receptor Ret, cadherin like domain 3 / Ret, cadherin like domain 1 / RET, cadherin-like domain 4 / : / RET Cadherin like domain 1 / RET Cadherin like domain 3 / RET Cadherin like domain 4 / RET, Cysteine Rich Domain / Cadherin domain ...Tyrosine-protein kinase, Ret receptor / Tyrosine-protein kinase receptor Ret, cadherin like domain 3 / Ret, cadherin like domain 1 / RET, cadherin-like domain 4 / : / RET Cadherin like domain 1 / RET Cadherin like domain 3 / RET Cadherin like domain 4 / RET, Cysteine Rich Domain / Cadherin domain / Cadherin-like / Cadherins domain profile. / Cadherin-like superfamily / : / Tyrosine-protein kinase, catalytic domain / Tyrosine kinase, catalytic domain / Tyrosine protein kinases specific active-site signature. / Tyrosine-protein kinase, active site / Serine-threonine/tyrosine-protein kinase, catalytic domain / Protein tyrosine and serine/threonine kinase / Protein kinase, ATP binding site / Protein kinases ATP-binding region signature. / Protein kinase domain profile. / Protein kinase domain / Protein kinase-like domain superfamilySimilarity search - Domain/homology |
|---|
| Biological species |  Homo sapiens (human) Homo sapiens (human) |
|---|
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 3.51 Å MOLECULAR REPLACEMENT / Resolution: 3.51 Å |
|---|
 Authors Authors | Lee, C.C. / Spraggon, G. |
|---|
| Funding support |  United States, 1items United States, 1items | Organization | Grant number | Country |
|---|
| Not funded | |  United States United States |
|
|---|
 Citation Citation |  Journal: Acs Med.Chem.Lett. / Year: 2021 Journal: Acs Med.Chem.Lett. / Year: 2021
Title: Antitarget Selectivity and Tolerability of Novel Pyrrolo[2,3- d ]pyrimidine RET Inhibitors.
Authors: Mathison, C.J.N. / Yang, Y. / Nelson, J. / Huang, Z. / Jiang, J. / Chianelli, D. / Rucker, P.V. / Roland, J. / Xie, Y.F. / Epple, R. / Bursulaya, B. / Lee, C. / Gao, M.Y. / Shaffer, J. / ...Authors: Mathison, C.J.N. / Yang, Y. / Nelson, J. / Huang, Z. / Jiang, J. / Chianelli, D. / Rucker, P.V. / Roland, J. / Xie, Y.F. / Epple, R. / Bursulaya, B. / Lee, C. / Gao, M.Y. / Shaffer, J. / Briones, S. / Sarkisova, Y. / Galkin, A. / Li, L. / Li, N. / Li, C. / Hua, S. / Kasibhatla, S. / Kinyamu-Akunda, J. / Kikkawa, R. / Molteni, V. / Tellew, J.E. |
|---|
| History | | Deposition | Aug 17, 2021 | Deposition site: RCSB / Processing site: RCSB |
|---|
| Revision 1.0 | Jan 19, 2022 | Provider: repository / Type: Initial release |
|---|
| Revision 1.1 | Oct 18, 2023 | Group: Data collection / Refinement description
Category: chem_comp_atom / chem_comp_bond / pdbx_initial_refinement_model |
|---|
| Revision 1.2 | Nov 15, 2023 | Group: Data collection / Category: chem_comp_atom / chem_comp_bond / Item: _chem_comp_atom.atom_id / _chem_comp_bond.atom_id_2 |
|---|
| Revision 1.3 | Nov 6, 2024 | Group: Structure summary / Category: pdbx_entry_details / pdbx_modification_feature / Item: _pdbx_entry_details.has_protein_modification |
|---|
|
|---|
 Yorodumi
Yorodumi Open data
Open data Basic information
Basic information Components
Components Keywords
Keywords Function and homology information
Function and homology information Homo sapiens (human)
Homo sapiens (human) X-RAY DIFFRACTION /
X-RAY DIFFRACTION /  SYNCHROTRON /
SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 3.51 Å
MOLECULAR REPLACEMENT / Resolution: 3.51 Å  Authors
Authors United States, 1items
United States, 1items  Citation
Citation Journal: Acs Med.Chem.Lett. / Year: 2021
Journal: Acs Med.Chem.Lett. / Year: 2021 Structure visualization
Structure visualization Molmil
Molmil Jmol/JSmol
Jmol/JSmol Downloads & links
Downloads & links Download
Download 7run.cif.gz
7run.cif.gz PDBx/mmCIF format
PDBx/mmCIF format pdb7run.ent.gz
pdb7run.ent.gz PDB format
PDB format 7run.json.gz
7run.json.gz PDBx/mmJSON format
PDBx/mmJSON format Other downloads
Other downloads 7run_validation.pdf.gz
7run_validation.pdf.gz wwPDB validaton report
wwPDB validaton report 7run_full_validation.pdf.gz
7run_full_validation.pdf.gz 7run_validation.xml.gz
7run_validation.xml.gz 7run_validation.cif.gz
7run_validation.cif.gz https://data.pdbj.org/pub/pdb/validation_reports/ru/7run
https://data.pdbj.org/pub/pdb/validation_reports/ru/7run ftp://data.pdbj.org/pub/pdb/validation_reports/ru/7run
ftp://data.pdbj.org/pub/pdb/validation_reports/ru/7run
 Links
Links Assembly
Assembly
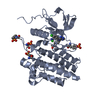

 Components
Components Homo sapiens (human) / Gene: RET, CDHF12, CDHR16, PTC, RET51 / Production host:
Homo sapiens (human) / Gene: RET, CDHF12, CDHR16, PTC, RET51 / Production host: 
 X-RAY DIFFRACTION / Number of used crystals: 1
X-RAY DIFFRACTION / Number of used crystals: 1  Sample preparation
Sample preparation SYNCHROTRON / Site:
SYNCHROTRON / Site:  ALS
ALS  / Beamline: 5.0.3 / Wavelength: 0.97648 Å
/ Beamline: 5.0.3 / Wavelength: 0.97648 Å Processing
Processing MOLECULAR REPLACEMENT
MOLECULAR REPLACEMENT Movie
Movie Controller
Controller












 PDBj
PDBj