[English] 日本語
 Yorodumi
Yorodumi- PDB-7q1c: Crystal structure of Trypanosoma cruzi histone deacetylase DAC2 c... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 7q1c | ||||||
|---|---|---|---|---|---|---|---|
| Title | Crystal structure of Trypanosoma cruzi histone deacetylase DAC2 complexed with a hydroxamate inhibitor | ||||||
 Components Components | Histone deacetylase DAC2 | ||||||
 Keywords Keywords | HYDROLASE / Epigenetics / Trypanosoma cruzi / Histone deacetylase / DAC2 / Pathogen | ||||||
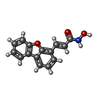
| Function / homology | Histone deacetylase domain / Arginase; Chain A / 3-Layer(aba) Sandwich / Alpha Beta / : / (E)-3-dibenzofuran-4-yl-N-oxidanyl-prop-2-enamide Function and homology information Function and homology information | ||||||
| Biological species |  | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.3 Å MOLECULAR REPLACEMENT / Resolution: 2.3 Å | ||||||
 Authors Authors | Ramos-Morales, E. / Marek, M. / Romier, C. | ||||||
| Funding support | European Union, 1items
| ||||||
 Citation Citation |  Journal: Cell Rep / Year: 2021 Journal: Cell Rep / Year: 2021Title: Species-selective targeting of pathogens revealed by the atypical structure and active site of Trypanosoma cruzi histone deacetylase DAC2. Authors: Marek, M. / Ramos-Morales, E. / Picchi-Constante, G.F.A. / Bayer, T. / Norstrom, C. / Herp, D. / Sales-Junior, P.A. / Guerra-Slompo, E.P. / Hausmann, K. / Chakrabarti, A. / Shaik, T.B. / ...Authors: Marek, M. / Ramos-Morales, E. / Picchi-Constante, G.F.A. / Bayer, T. / Norstrom, C. / Herp, D. / Sales-Junior, P.A. / Guerra-Slompo, E.P. / Hausmann, K. / Chakrabarti, A. / Shaik, T.B. / Merz, A. / Troesch, E. / Schmidtkunz, K. / Goldenberg, S. / Pierce, R.J. / Mourao, M.M. / Jung, M. / Schultz, J. / Sippl, W. / Zanchin, N.I.T. / Romier, C. #1:  Journal: Acta Crystallogr., Sect. D: Biol. Crystallogr. / Year: 2012 Journal: Acta Crystallogr., Sect. D: Biol. Crystallogr. / Year: 2012Title: Towards automated crystallographic structure refinement with phenix.refine. Authors: Afonine, P.V. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  7q1c.cif.gz 7q1c.cif.gz | 402.9 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb7q1c.ent.gz pdb7q1c.ent.gz | 273.7 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  7q1c.json.gz 7q1c.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/q1/7q1c https://data.pdbj.org/pub/pdb/validation_reports/q1/7q1c ftp://data.pdbj.org/pub/pdb/validation_reports/q1/7q1c ftp://data.pdbj.org/pub/pdb/validation_reports/q1/7q1c | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  7q1bC  1t67S S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||||||
| 2 | 
| ||||||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 50408.746 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   #2: Chemical | #3: Chemical | ChemComp-K / #4: Chemical | #5: Water | ChemComp-HOH / | Has ligand of interest | Y | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.61 Å3/Da / Density % sol: 52.9 % |
|---|---|
| Crystal grow | Temperature: 293 K / Method: vapor diffusion, sitting drop Details: 7.5%(W/V) PEG 8K, 0.1 M Bis-tris pH 6.3, 0.2 M MgCl2 |
-Data collection
| Diffraction | Mean temperature: 100 K / Serial crystal experiment: N |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  SOLEIL SOLEIL  / Beamline: PROXIMA 2 / Wavelength: 0.979957 Å / Beamline: PROXIMA 2 / Wavelength: 0.979957 Å |
| Detector | Type: DECTRIS EIGER X 9M / Detector: PIXEL / Date: May 26, 2018 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.979957 Å / Relative weight: 1 |
| Reflection | Resolution: 2.3→50 Å / Num. obs: 52026 / % possible obs: 99.7 % / Redundancy: 7 % / Biso Wilson estimate: 36.15 Å2 / CC1/2: 0.995 / Rmerge(I) obs: 0.195 / Net I/σ(I): 7.76 |
| Reflection shell | Resolution: 2.3→2.44 Å / Redundancy: 6.78 % / Rmerge(I) obs: 1.687 / Mean I/σ(I) obs: 1.23 / Num. unique obs: 8289 / CC1/2: 0.425 / % possible all: 98.8 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 1T67 Resolution: 2.3→48.11 Å / SU ML: 0.3138 / Cross valid method: FREE R-VALUE / σ(F): 0.01 / Phase error: 27.335 Stereochemistry target values: GeoStd + Monomer Library + CDL v1.2
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å / Solvent model: FLAT BULK SOLVENT MODEL | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 45.52 Å2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.3→48.11 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Refine-ID: X-RAY DIFFRACTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group | Refine-ID: X-RAY DIFFRACTION / Auth seq-ID: 13 - 700 / Label seq-ID: 1
|
 Movie
Movie Controller
Controller











 PDBj
PDBj