+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 7plq | ||||||
|---|---|---|---|---|---|---|---|
| Title | Crystal structure of the PARP domain of wheat SRO1 | ||||||
 Components Components | SIMILAR TO RCD1 (SRO1) | ||||||
 Keywords Keywords | PLANT PROTEIN / poly(ADP-ribose)-polymerase (PARP) domain | ||||||
| Function / homology |  Function and homology information Function and homology information | ||||||
| Biological species |  | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  SAD / Resolution: 2.13 Å SAD / Resolution: 2.13 Å | ||||||
 Authors Authors | Wirthmueller, L. / Loll, B. | ||||||
| Funding support |  Germany, 1items Germany, 1items
| ||||||
 Citation Citation |  Journal: Plant Cell / Year: 2022 Journal: Plant Cell / Year: 2022Title: The superior salinity tolerance of bread wheat cultivar Shanrong No. 3 is unlikely to be caused by elevated Ta-sro1 poly-(ADP-ribose) polymerase activity. Authors: Vogt, S. / Feijs, K. / Hosch, S. / De Masi, R. / Lintermann, R. / Loll, B. / Wirthmueller, L. #1:  Journal: Biorxiv / Year: 2021 Journal: Biorxiv / Year: 2021Title: The superior salinity tolerance of wheat cultivar Shanrong No. 3 cannot be attributed to elevated Ta-sro1 poly(ADP-ribose) polymerase activity Authors: Vogt, S. / Feijs, K. / Hosch, S. / Lintermann, R. / Loll, B. / Wirthmueller, L. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  7plq.cif.gz 7plq.cif.gz | 163.6 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb7plq.ent.gz pdb7plq.ent.gz | 128 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  7plq.json.gz 7plq.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/pl/7plq https://data.pdbj.org/pub/pdb/validation_reports/pl/7plq ftp://data.pdbj.org/pub/pdb/validation_reports/pl/7plq ftp://data.pdbj.org/pub/pdb/validation_reports/pl/7plq | HTTPS FTP |
|---|
-Related structure data
| Similar structure data |
|---|
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 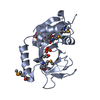
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2 | 
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Unit cell |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Noncrystallographic symmetry (NCS) | NCS domain:
NCS domain segments: Ens-ID: 1
|
 Movie
Movie Controller
Controller











 PDBj
PDBj