+ データを開く
データを開く
- 基本情報
基本情報
| 登録情報 | データベース: PDB / ID: 7lrd | ||||||
|---|---|---|---|---|---|---|---|
| タイトル | Cryo-EM of the SLFN12-PDE3A complex: Consensus subset model | ||||||
 要素 要素 |
| ||||||
 キーワード キーワード | RNA BINDING PROTEIN / complex / velcrin / molecular glue / DNMDP | ||||||
| 機能・相同性 |  機能・相同性情報 機能・相同性情報3',5'-cGMP-inhibited cyclic-nucleotide phosphodiesterase activity / estrogen binding / positive regulation of oocyte development / regulation of ribonuclease activity / regulation of meiotic nuclear division / cellular response to cGMP / rRNA catabolic process / negative regulation of adenylate cyclase-activating G protein-coupled receptor signaling pathway / positive regulation of vascular permeability / negative regulation of vascular permeability ...3',5'-cGMP-inhibited cyclic-nucleotide phosphodiesterase activity / estrogen binding / positive regulation of oocyte development / regulation of ribonuclease activity / regulation of meiotic nuclear division / cellular response to cGMP / rRNA catabolic process / negative regulation of adenylate cyclase-activating G protein-coupled receptor signaling pathway / positive regulation of vascular permeability / negative regulation of vascular permeability / negative regulation of cAMP/PKA signal transduction / oocyte maturation / : / 3',5'-cyclic-nucleotide phosphodiesterase / nuclear estrogen receptor activity / 3',5'-cyclic-nucleotide phosphodiesterase activity / 3',5'-cyclic-GMP phosphodiesterase activity / 3',5'-cyclic-AMP phosphodiesterase activity / RNA nuclease activity / cellular response to transforming growth factor beta stimulus / : / apoptotic signaling pathway / lipid metabolic process / ribosome binding / G alpha (s) signalling events / 加水分解酵素; エステル加水分解酵素 / G protein-coupled receptor signaling pathway / response to xenobiotic stimulus / negative regulation of apoptotic process / metal ion binding / nucleus / membrane / cytosol 類似検索 - 分子機能 | ||||||
| 生物種 |  Homo sapiens (ヒト) Homo sapiens (ヒト) | ||||||
| 手法 | 電子顕微鏡法 / 単粒子再構成法 / クライオ電子顕微鏡法 / 解像度: 3.22 Å | ||||||
 データ登録者 データ登録者 | Fuller, J.R. / Garvie, C.W. / Lemke, C.T. | ||||||
 引用 引用 |  ジャーナル: Nat Commun / 年: 2021 ジャーナル: Nat Commun / 年: 2021タイトル: Structure of PDE3A-SLFN12 complex reveals requirements for activation of SLFN12 RNase. 著者: Colin W Garvie / Xiaoyun Wu / Malvina Papanastasiou / Sooncheol Lee / James Fuller / Gavin R Schnitzler / Steven W Horner / Andrew Baker / Terry Zhang / James P Mullahoo / Lindsay Westlake / ...著者: Colin W Garvie / Xiaoyun Wu / Malvina Papanastasiou / Sooncheol Lee / James Fuller / Gavin R Schnitzler / Steven W Horner / Andrew Baker / Terry Zhang / James P Mullahoo / Lindsay Westlake / Stephanie H Hoyt / Marcus Toetzl / Matthew J Ranaghan / Luc de Waal / Joseph McGaunn / Bethany Kaplan / Federica Piccioni / Xiaoping Yang / Martin Lange / Adrian Tersteegen / Donald Raymond / Timothy A Lewis / Steven A Carr / Andrew D Cherniack / Christopher T Lemke / Matthew Meyerson / Heidi Greulich /   要旨: DNMDP and related compounds, or velcrins, induce complex formation between the phosphodiesterase PDE3A and the SLFN12 protein, leading to a cytotoxic response in cancer cells that express elevated ...DNMDP and related compounds, or velcrins, induce complex formation between the phosphodiesterase PDE3A and the SLFN12 protein, leading to a cytotoxic response in cancer cells that express elevated levels of both proteins. The mechanisms by which velcrins induce complex formation, and how the PDE3A-SLFN12 complex causes cancer cell death, are not fully understood. Here, we show that PDE3A and SLFN12 form a heterotetramer stabilized by binding of DNMDP. Interactions between the C-terminal alpha helix of SLFN12 and residues near the active site of PDE3A are required for complex formation, and are further stabilized by interactions between SLFN12 and DNMDP. Moreover, we demonstrate that SLFN12 is an RNase, that PDE3A binding increases SLFN12 RNase activity, and that SLFN12 RNase activity is required for DNMDP response. This new mechanistic understanding will facilitate development of velcrin compounds into new cancer therapies. | ||||||
| 履歴 |
|
- 構造の表示
構造の表示
| ムービー |
 ムービービューア ムービービューア |
|---|---|
| 構造ビューア | 分子:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
- ダウンロードとリンク
ダウンロードとリンク
- ダウンロード
ダウンロード
| PDBx/mmCIF形式 |  7lrd.cif.gz 7lrd.cif.gz | 331.6 KB | 表示 |  PDBx/mmCIF形式 PDBx/mmCIF形式 |
|---|---|---|---|---|
| PDB形式 |  pdb7lrd.ent.gz pdb7lrd.ent.gz | 260.7 KB | 表示 |  PDB形式 PDB形式 |
| PDBx/mmJSON形式 |  7lrd.json.gz 7lrd.json.gz | ツリー表示 |  PDBx/mmJSON形式 PDBx/mmJSON形式 | |
| その他 |  その他のダウンロード その他のダウンロード |
-検証レポート
| 文書・要旨 |  7lrd_validation.pdf.gz 7lrd_validation.pdf.gz | 1.4 MB | 表示 |  wwPDB検証レポート wwPDB検証レポート |
|---|---|---|---|---|
| 文書・詳細版 |  7lrd_full_validation.pdf.gz 7lrd_full_validation.pdf.gz | 1.4 MB | 表示 | |
| XML形式データ |  7lrd_validation.xml.gz 7lrd_validation.xml.gz | 61.3 KB | 表示 | |
| CIF形式データ |  7lrd_validation.cif.gz 7lrd_validation.cif.gz | 90.8 KB | 表示 | |
| アーカイブディレクトリ |  https://data.pdbj.org/pub/pdb/validation_reports/lr/7lrd https://data.pdbj.org/pub/pdb/validation_reports/lr/7lrd ftp://data.pdbj.org/pub/pdb/validation_reports/lr/7lrd ftp://data.pdbj.org/pub/pdb/validation_reports/lr/7lrd | HTTPS FTP |
-関連構造データ
- リンク
リンク
- 集合体
集合体
| 登録構造単位 | 
|
|---|---|
| 1 |
|
- 要素
要素
-タンパク質 , 2種, 4分子 ACBD
| #1: タンパク質 | 分子量: 67383.734 Da / 分子数: 2 / 由来タイプ: 組換発現 / 由来: (組換発現)  Homo sapiens (ヒト) / 遺伝子: SLFN12 / 発現宿主: Homo sapiens (ヒト) / 遺伝子: SLFN12 / 発現宿主:  unidentified baculovirus (ウイルス) / 参照: UniProt: Q8IYM2 unidentified baculovirus (ウイルス) / 参照: UniProt: Q8IYM2#2: タンパク質 | 分子量: 57352.996 Da / 分子数: 2 / 由来タイプ: 組換発現 / 由来: (組換発現)  Homo sapiens (ヒト) / 遺伝子: PDE3A / 発現宿主: Homo sapiens (ヒト) / 遺伝子: PDE3A / 発現宿主:  unidentified baculovirus (ウイルス) unidentified baculovirus (ウイルス)参照: UniProt: Q14432, 3',5'-cyclic-nucleotide phosphodiesterase |
|---|
-非ポリマー , 4種, 8分子 


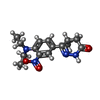



| #3: 化合物 | | #4: 化合物 | #5: 化合物 | #6: 化合物 | |
|---|
-詳細
| 研究の焦点であるリガンドがあるか | Y |
|---|
-実験情報
-実験
| 実験 | 手法: 電子顕微鏡法 |
|---|---|
| EM実験 | 試料の集合状態: PARTICLE / 3次元再構成法: 単粒子再構成法 |
- 試料調製
試料調製
| 構成要素 | 名称: SLFN12-PDE3A complex, consensus subset model / タイプ: COMPLEX / Entity ID: #1-#2 / 由来: RECOMBINANT | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 分子量 | 単位: MEGADALTONS / 実験値: NO | |||||||||||||||||||||||||
| 由来(天然) | 生物種:  Homo sapiens (ヒト) Homo sapiens (ヒト) | |||||||||||||||||||||||||
| 由来(組換発現) | 生物種:  | |||||||||||||||||||||||||
| 緩衝液 | pH: 7.4 詳細: 0.0038% NP-40s detergent (CAS 9016-45-9) added immediately prior to plunge freezing | |||||||||||||||||||||||||
| 緩衝液成分 |
| |||||||||||||||||||||||||
| 試料 | 濃度: 0.6 mg/ml / 包埋: NO / シャドウイング: NO / 染色: NO / 凍結: YES | |||||||||||||||||||||||||
| 試料支持 | グリッドの材料: GOLD / グリッドのサイズ: 400 divisions/in. / グリッドのタイプ: C-flat | |||||||||||||||||||||||||
| 急速凍結 | 装置: FEI VITROBOT MARK IV / 凍結剤: ETHANE / 湿度: 100 % / 凍結前の試料温度: 291 K / 詳細: 4.5 second blot time |
- 電子顕微鏡撮影
電子顕微鏡撮影
| 実験機器 |  モデル: Titan Krios / 画像提供: FEI Company |
|---|---|
| 顕微鏡 | モデル: FEI TITAN KRIOS |
| 電子銃 | 電子線源:  FIELD EMISSION GUN / 加速電圧: 300 kV / 照射モード: FLOOD BEAM FIELD EMISSION GUN / 加速電圧: 300 kV / 照射モード: FLOOD BEAM |
| 電子レンズ | モード: BRIGHT FIELD / 倍率(公称値): 81000 X / Cs: 2.7 mm / C2レンズ絞り径: 70 µm / アライメント法: ZEMLIN TABLEAU |
| 試料ホルダ | 凍結剤: NITROGEN 試料ホルダーモデル: FEI TITAN KRIOS AUTOGRID HOLDER |
| 撮影 | 電子線照射量: 50 e/Å2 フィルム・検出器のモデル: GATAN K3 BIOQUANTUM (6k x 4k) 撮影したグリッド数: 1 / 実像数: 3163 詳細: Exposures were collected in super-resolution mode, as movies fractionated over 40 frames |
| 画像スキャン | 横: 5760 / 縦: 4092 |
- 解析
解析
| EMソフトウェア |
| ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CTF補正 | 詳細: CTF correction was applied throughout the entire processing workflow, beginning with particle picking タイプ: PHASE FLIPPING AND AMPLITUDE CORRECTION | ||||||||||||||||||||||||||||||||||||
| 粒子像の選択 | 選択した粒子像数: 2160426 | ||||||||||||||||||||||||||||||||||||
| 対称性 | 点対称性: C2 (2回回転対称) | ||||||||||||||||||||||||||||||||||||
| 3次元再構成 | 解像度: 3.22 Å / 解像度の算出法: FSC 0.143 CUT-OFF / 粒子像の数: 47632 / アルゴリズム: FOURIER SPACE / 詳細: Relion 3D refinement / 対称性のタイプ: POINT | ||||||||||||||||||||||||||||||||||||
| 原子モデル構築 | 空間: REAL 詳細: The model was generated by rigid body fitting of the SLFN12 and PDE3A bodies (from multi-body refinement and deposited separately) into this map using UCSF Chimera and Coot, followed by local ...詳細: The model was generated by rigid body fitting of the SLFN12 and PDE3A bodies (from multi-body refinement and deposited separately) into this map using UCSF Chimera and Coot, followed by local refinement in Coot of the linkers between the bodies to ameliorate backbone geometry. | ||||||||||||||||||||||||||||||||||||
| 原子モデル構築 | PDB-ID: 5YD0 Accession code: 5YD0 / Source name: PDB / タイプ: experimental model |
 ムービー
ムービー コントローラー
コントローラー



















 PDBj
PDBj





