[English] 日本語
 Yorodumi
Yorodumi- PDB-7kwe: Crystal structure of the catalytic domain of human PDE3A bound to... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 7kwe | ||||||
|---|---|---|---|---|---|---|---|
| Title | Crystal structure of the catalytic domain of human PDE3A bound to DNMDP | ||||||
 Components Components | cGMP-inhibited 3',5'-cyclic phosphodiesterase A | ||||||
 Keywords Keywords | HYDROLASE | ||||||
| Function / homology |  Function and homology information Function and homology information3',5'-cGMP-inhibited cyclic-nucleotide phosphodiesterase activity / estrogen binding / positive regulation of oocyte development / regulation of ribonuclease activity / regulation of meiotic nuclear division / cellular response to cGMP / negative regulation of adenylate cyclase-activating G protein-coupled receptor signaling pathway / positive regulation of vascular permeability / negative regulation of vascular permeability / oocyte maturation ...3',5'-cGMP-inhibited cyclic-nucleotide phosphodiesterase activity / estrogen binding / positive regulation of oocyte development / regulation of ribonuclease activity / regulation of meiotic nuclear division / cellular response to cGMP / negative regulation of adenylate cyclase-activating G protein-coupled receptor signaling pathway / positive regulation of vascular permeability / negative regulation of vascular permeability / oocyte maturation / : / : / 3',5'-cyclic-nucleotide phosphodiesterase / nuclear estrogen receptor activity / 3',5'-cyclic-nucleotide phosphodiesterase activity / 3',5'-cyclic-GMP phosphodiesterase activity / 3',5'-cyclic-AMP phosphodiesterase activity / negative regulation of cAMP/PKA signal transduction / cellular response to transforming growth factor beta stimulus / apoptotic signaling pathway / lipid metabolic process / G alpha (s) signalling events / G protein-coupled receptor signaling pathway / response to xenobiotic stimulus / negative regulation of apoptotic process / metal ion binding / membrane / cytosol Similarity search - Function | ||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2 Å MOLECULAR REPLACEMENT / Resolution: 2 Å | ||||||
 Authors Authors | Horner, S.W. / Garvie, C. | ||||||
 Citation Citation |  Journal: Nat Commun / Year: 2021 Journal: Nat Commun / Year: 2021Title: Structure of PDE3A-SLFN12 complex reveals requirements for activation of SLFN12 RNase. Authors: Colin W Garvie / Xiaoyun Wu / Malvina Papanastasiou / Sooncheol Lee / James Fuller / Gavin R Schnitzler / Steven W Horner / Andrew Baker / Terry Zhang / James P Mullahoo / Lindsay Westlake / ...Authors: Colin W Garvie / Xiaoyun Wu / Malvina Papanastasiou / Sooncheol Lee / James Fuller / Gavin R Schnitzler / Steven W Horner / Andrew Baker / Terry Zhang / James P Mullahoo / Lindsay Westlake / Stephanie H Hoyt / Marcus Toetzl / Matthew J Ranaghan / Luc de Waal / Joseph McGaunn / Bethany Kaplan / Federica Piccioni / Xiaoping Yang / Martin Lange / Adrian Tersteegen / Donald Raymond / Timothy A Lewis / Steven A Carr / Andrew D Cherniack / Christopher T Lemke / Matthew Meyerson / Heidi Greulich /   Abstract: DNMDP and related compounds, or velcrins, induce complex formation between the phosphodiesterase PDE3A and the SLFN12 protein, leading to a cytotoxic response in cancer cells that express elevated ...DNMDP and related compounds, or velcrins, induce complex formation between the phosphodiesterase PDE3A and the SLFN12 protein, leading to a cytotoxic response in cancer cells that express elevated levels of both proteins. The mechanisms by which velcrins induce complex formation, and how the PDE3A-SLFN12 complex causes cancer cell death, are not fully understood. Here, we show that PDE3A and SLFN12 form a heterotetramer stabilized by binding of DNMDP. Interactions between the C-terminal alpha helix of SLFN12 and residues near the active site of PDE3A are required for complex formation, and are further stabilized by interactions between SLFN12 and DNMDP. Moreover, we demonstrate that SLFN12 is an RNase, that PDE3A binding increases SLFN12 RNase activity, and that SLFN12 RNase activity is required for DNMDP response. This new mechanistic understanding will facilitate development of velcrin compounds into new cancer therapies. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  7kwe.cif.gz 7kwe.cif.gz | 611.5 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb7kwe.ent.gz pdb7kwe.ent.gz | 505.4 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  7kwe.json.gz 7kwe.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/kw/7kwe https://data.pdbj.org/pub/pdb/validation_reports/kw/7kwe ftp://data.pdbj.org/pub/pdb/validation_reports/kw/7kwe ftp://data.pdbj.org/pub/pdb/validation_reports/kw/7kwe | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  7l27C  7l28C  7l29C  7lrcC  7lrdC  7lreC  1so2S S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2 | 
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Unit cell |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Noncrystallographic symmetry (NCS) | NCS domain:
NCS domain segments: Component-ID: _ / Refine code: _
NCS ensembles :
|
- Components
Components
-Protein , 1 types, 4 molecules ADCB
| #1: Protein | Mass: 43169.121 Da / Num. of mol.: 4 / Fragment: Catalytic domain Source method: isolated from a genetically manipulated source Details: N-terminal glycine from cleavage with TEV protease. Residues 780 to 800 replaced with GGSGGS linker. Residues 1029 to 1067 replaced with GGSGGS linker. Source: (gene. exp.)  Homo sapiens (human) / Gene: PDE3A / Plasmid: pET21 / Production host: Homo sapiens (human) / Gene: PDE3A / Plasmid: pET21 / Production host:  References: UniProt: Q14432, 3',5'-cyclic-nucleotide phosphodiesterase |
|---|
-Non-polymers , 5 types, 375 molecules 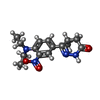








| #2: Chemical | ChemComp-X5M / ( #3: Chemical | ChemComp-MN / #4: Chemical | ChemComp-MG / #5: Chemical | ChemComp-ACT / #6: Water | ChemComp-HOH / | |
|---|
-Details
| Has ligand of interest | Y |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.2 Å3/Da / Density % sol: 44.01 % |
|---|---|
| Crystal grow | Temperature: 293.15 K / Method: vapor diffusion, hanging drop / pH: 5.9 / Details: MES, PEG 3350, calcium acetate / PH range: 5.7 - 6.1 |
-Data collection
| Diffraction | Mean temperature: 100 K / Serial crystal experiment: N |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  ESRF ESRF  / Beamline: ID30B / Wavelength: 0.976251 Å / Beamline: ID30B / Wavelength: 0.976251 Å |
| Detector | Type: DECTRIS PILATUS3 S 6M / Detector: PIXEL / Date: Jun 1, 2018 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.976251 Å / Relative weight: 1 |
| Reflection | Resolution: 2→47.7 Å / Num. obs: 187181 / % possible obs: 94.3 % / Redundancy: 1.8 % / CC1/2: 0.998 / Rrim(I) all: 0.057 / Net I/σ(I): 10.79 |
| Reflection shell | Resolution: 2→2.11 Å / Redundancy: 1.7 % / Mean I/σ(I) obs: 1.47 / Num. unique obs: 29075 / CC1/2: 0.64 / Rrim(I) all: 0.747 / % possible all: 91.7 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 1SO2 Resolution: 2→47.7 Å / Cor.coef. Fo:Fc: 0.966 / Cor.coef. Fo:Fc free: 0.956 / SU B: 10.709 / SU ML: 0.144 / Cross valid method: THROUGHOUT / σ(F): 0 / ESU R: 0.194 / ESU R Free: 0.161 / Stereochemistry target values: MAXIMUM LIKELIHOOD Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS U VALUES : WITH TLS ADDED
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.2 Å / Solvent model: MASK | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso max: 138.33 Å2 / Biso mean: 49.716 Å2 / Biso min: 23.67 Å2
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: final / Resolution: 2→47.7 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints NCS | Refine-ID: X-RAY DIFFRACTION / Type: interatomic distance / Weight position: 0.05
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 2.002→2.054 Å / Rfactor Rfree error: 0 / Total num. of bins used: 20
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Refine-ID: X-RAY DIFFRACTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group |
|
 Movie
Movie Controller
Controller














 PDBj
PDBj




