[English] 日本語
 Yorodumi
Yorodumi- PDB-7l7g: Electron cryo-microscopy of the eukaryotic translation initiation... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 7l7g | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Electron cryo-microscopy of the eukaryotic translation initiation factor 2B from Homo sapiens (updated model of PDB ID: 6CAJ) | |||||||||
 Components Components | (Translation initiation factor eIF-2B subunit ...) x 5 | |||||||||
 Keywords Keywords | TRANSLATION / Integrated stress response | |||||||||
| Function / homology |  Function and homology information Function and homology informationeukaryotic translation initiation factor 2B complex / Recycling of eIF2:GDP / cytoplasmic translational initiation / astrocyte development / guanyl-nucleotide exchange factor complex / astrocyte differentiation / oligodendrocyte development / regulation of translational initiation / positive regulation of translational initiation / response to glucose ...eukaryotic translation initiation factor 2B complex / Recycling of eIF2:GDP / cytoplasmic translational initiation / astrocyte development / guanyl-nucleotide exchange factor complex / astrocyte differentiation / oligodendrocyte development / regulation of translational initiation / positive regulation of translational initiation / response to glucose / ovarian follicle development / myelination / translation initiation factor binding / translation initiation factor activity / guanyl-nucleotide exchange factor activity / response to endoplasmic reticulum stress / central nervous system development / hippocampus development / translational initiation / response to peptide hormone / T cell receptor signaling pathway / regulation of translation / response to heat / positive regulation of apoptotic process / GTP binding / ATP binding / identical protein binding / nucleus / membrane / plasma membrane / cytosol / cytoplasm Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | ELECTRON MICROSCOPY / single particle reconstruction / cryo EM / Resolution: 3 Å | |||||||||
 Authors Authors | Tsai, J.C. / Miller-Vedam, L.E. / Anand, A. / Jaishankar, P. / Nguyen, H.C. / Wang, L. / Renslo, A.R. / Frost, A. / Walter, P. | |||||||||
| Funding support |  United States, 2items United States, 2items
| |||||||||
 Citation Citation |  Journal: Elife / Year: 2021 Journal: Elife / Year: 2021Title: eIF2B conformation and assembly state regulate the integrated stress response. Authors: Michael Schoof / Morgane Boone / Lan Wang / Rosalie Lawrence / Adam Frost / Peter Walter /  Abstract: The integrated stress response (ISR) is activated by phosphorylation of the translation initiation factor eIF2 in response to various stress conditions. Phosphorylated eIF2 (eIF2-P) inhibits eIF2's ...The integrated stress response (ISR) is activated by phosphorylation of the translation initiation factor eIF2 in response to various stress conditions. Phosphorylated eIF2 (eIF2-P) inhibits eIF2's nucleotide exchange factor eIF2B, a twofold symmetric heterodecamer assembled from subcomplexes. Here, we monitor and manipulate eIF2B assembly in vitro and in vivo. In the absence of eIF2B's α-subunit, the ISR is induced because unassembled eIF2B tetramer subcomplexes accumulate in cells. Upon addition of the small-molecule ISR inhibitor ISRIB, eIF2B tetramers assemble into active octamers. Surprisingly, ISRIB inhibits the ISR even in the context of fully assembled eIF2B decamers, revealing allosteric communication between the physically distant eIF2, eIF2-P, and ISRIB binding sites. Cryo-electron microscopy structures suggest a rocking motion in eIF2B that couples these binding sites. eIF2-P binding converts eIF2B decamers into 'conjoined tetramers' with diminished substrate binding and enzymatic activity. Canonical eIF2-P-driven ISR activation thus arises due to this change in eIF2B's conformational state. #1:  Journal: Science / Year: 2018 Journal: Science / Year: 2018Title: Structure of the nucleotide exchange factor eIF2B reveals mechanism of memory-enhancing molecule. Authors: Jordan C Tsai / Lakshmi E Miller-Vedam / Aditya A Anand / Priyadarshini Jaishankar / Henry C Nguyen / Adam R Renslo / Adam Frost / Peter Walter /  Abstract: Regulation by the integrated stress response (ISR) converges on the phosphorylation of translation initiation factor eIF2 in response to a variety of stresses. Phosphorylation converts eIF2 from a ...Regulation by the integrated stress response (ISR) converges on the phosphorylation of translation initiation factor eIF2 in response to a variety of stresses. Phosphorylation converts eIF2 from a substrate to a competitive inhibitor of its dedicated guanine nucleotide exchange factor, eIF2B, thereby inhibiting translation. ISRIB, a drug-like eIF2B activator, reverses the effects of eIF2 phosphorylation, and in rodents it enhances cognition and corrects cognitive deficits after brain injury. To determine its mechanism of action, we solved an atomic-resolution structure of ISRIB bound in a deep cleft within decameric human eIF2B by cryo-electron microscopy. Formation of fully active, decameric eIF2B holoenzyme depended on the assembly of two identical tetrameric subcomplexes, and ISRIB promoted this step by cross-bridging a central symmetry interface. Thus, regulation of eIF2B assembly emerges as a rheostat for eIF2B activity that tunes translation during the ISR and that can be further modulated by ISRIB. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  7l7g.cif.gz 7l7g.cif.gz | 583.2 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb7l7g.ent.gz pdb7l7g.ent.gz | 455.5 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  7l7g.json.gz 7l7g.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/l7/7l7g https://data.pdbj.org/pub/pdb/validation_reports/l7/7l7g ftp://data.pdbj.org/pub/pdb/validation_reports/l7/7l7g ftp://data.pdbj.org/pub/pdb/validation_reports/l7/7l7g | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  7443M  7l70C M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
-Translation initiation factor eIF-2B subunit ... , 5 types, 10 molecules BAJICDFEHG
| #1: Protein | Mass: 80452.586 Da / Num. of mol.: 2 / Mutation: I587V Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: EIF2B5, EIF2BE / Production host: Homo sapiens (human) / Gene: EIF2B5, EIF2BE / Production host:  #2: Protein | Mass: 50304.230 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: EIF2B3 / Production host: Homo sapiens (human) / Gene: EIF2B3 / Production host:  #3: Protein | Mass: 41008.578 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: EIF2B2, EIF2BB / Production host: Homo sapiens (human) / Gene: EIF2B2, EIF2BB / Production host:  #4: Protein | Mass: 57640.168 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: EIF2B4, EIF2BD / Production host: Homo sapiens (human) / Gene: EIF2B4, EIF2BD / Production host:  #5: Protein | Mass: 33754.148 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: EIF2B1, EIF2BA / Production host: Homo sapiens (human) / Gene: EIF2B1, EIF2BA / Production host:  |
|---|
-Non-polymers , 1 types, 1 molecules 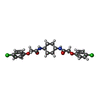
| #6: Chemical | ChemComp-C7B / |
|---|
-Details
| Has ligand of interest | Y |
|---|
-Experimental details
-Experiment
| Experiment | Method: ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method: single particle reconstruction |
- Sample preparation
Sample preparation
| Component | Name: the eukaryotic translation initiation factor 2B from Homo sapiens Type: COMPLEX / Entity ID: #1-#5 / Source: RECOMBINANT |
|---|---|
| Molecular weight | Value: 0.5 MDa / Experimental value: YES |
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Source (recombinant) | Organism:  |
| Buffer solution | pH: 7.5 |
| Specimen | Embedding applied: NO / Shadowing applied: NO / Staining applied: NO / Vitrification applied: YES |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: FEI TITAN KRIOS |
| Electron gun | Electron source:  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD |
| Image recording | Electron dose: 80 e/Å2 / Film or detector model: GATAN K2 SUMMIT (4k x 4k) |
- Processing
Processing
| CTF correction | Type: PHASE FLIPPING AND AMPLITUDE CORRECTION |
|---|---|
| 3D reconstruction | Resolution: 3 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 202125 / Symmetry type: POINT |
 Movie
Movie Controller
Controller










 PDBj
PDBj






