[English] 日本語
 Yorodumi
Yorodumi- PDB-7aqg: Crystal Structure of Small Molecule Inhibitor TM5484 Bound to Sta... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 7aqg | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Crystal Structure of Small Molecule Inhibitor TM5484 Bound to Stabilized Active Plasminogen Activator Inhibitor-1 (PAI-1-W175F) | |||||||||
 Components Components |
| |||||||||
 Keywords Keywords | HYDROLASE / plasminogen activator inhibitor-1 / PAI-1 / PAI-1-W175F / serpin / protease inhibitor / serine protease inhibitor / small molecule / antibody fragments / nanobodies / protein complex | |||||||||
| Function / homology |  Function and homology information Function and homology informationpositive regulation of leukotriene production involved in inflammatory response / dentinogenesis / negative regulation of smooth muscle cell-matrix adhesion / peptidase inhibitor complex / positive regulation of coagulation / negative regulation of smooth muscle cell migration / Regulation of MITF-M-dependent genes involved in extracellular matrix, focal adhesion and epithelial-to-mesenchymal transition / negative regulation of vascular wound healing / negative regulation of wound healing / positive regulation of odontoblast differentiation ...positive regulation of leukotriene production involved in inflammatory response / dentinogenesis / negative regulation of smooth muscle cell-matrix adhesion / peptidase inhibitor complex / positive regulation of coagulation / negative regulation of smooth muscle cell migration / Regulation of MITF-M-dependent genes involved in extracellular matrix, focal adhesion and epithelial-to-mesenchymal transition / negative regulation of vascular wound healing / negative regulation of wound healing / positive regulation of odontoblast differentiation / negative regulation of cell adhesion mediated by integrin / regulation of signaling receptor activity / negative regulation of plasminogen activation / Dissolution of Fibrin Clot / positive regulation of monocyte chemotaxis / replicative senescence / negative regulation of blood coagulation / positive regulation of blood coagulation / negative regulation of fibrinolysis / ECM proteoglycans / negative regulation of endothelial cell apoptotic process / negative regulation of extrinsic apoptotic signaling pathway via death domain receptors / serine protease inhibitor complex / fibrinolysis / negative regulation of proteolysis / BMAL1:CLOCK,NPAS2 activates circadian expression / platelet alpha granule lumen / negative regulation of cell migration / positive regulation of interleukin-8 production / serine-type endopeptidase inhibitor activity / SMAD2/SMAD3:SMAD4 heterotrimer regulates transcription / positive regulation of receptor-mediated endocytosis / positive regulation of angiogenesis / positive regulation of inflammatory response / Platelet degranulation / : / cellular response to lipopolysaccharide / protease binding / angiogenesis / defense response to Gram-negative bacterium / signaling receptor binding / extracellular space / extracellular exosome / extracellular region / plasma membrane Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.27 Å MOLECULAR REPLACEMENT / Resolution: 2.27 Å | |||||||||
 Authors Authors | Sillen, M. / Strelkov, S.V. / Declerck, P.J. | |||||||||
| Funding support |  Belgium, 2items Belgium, 2items
| |||||||||
 Citation Citation |  Journal: Int J Mol Sci / Year: 2021 Journal: Int J Mol Sci / Year: 2021Title: Structural Insight into the Two-Step Mechanism of PAI-1 Inhibition by Small Molecule TM5484. Authors: Sillen, M. / Miyata, T. / Vaughan, D.E. / Strelkov, S.V. / Declerck, P.J. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  7aqg.cif.gz 7aqg.cif.gz | 130.5 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb7aqg.ent.gz pdb7aqg.ent.gz | 98.5 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  7aqg.json.gz 7aqg.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/aq/7aqg https://data.pdbj.org/pub/pdb/validation_reports/aq/7aqg ftp://data.pdbj.org/pub/pdb/validation_reports/aq/7aqg ftp://data.pdbj.org/pub/pdb/validation_reports/aq/7aqg | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  7aqfC  6gwnS S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 42780.031 Da / Num. of mol.: 1 / Mutation: W175F Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: SERPINE1, PAI1, PLANH1 / Plasmid: pETHSUK2 / Production host: Homo sapiens (human) / Gene: SERPINE1, PAI1, PLANH1 / Plasmid: pETHSUK2 / Production host:  |
|---|---|
| #2: Antibody | Mass: 12752.254 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   |
| #3: Antibody | Mass: 13087.387 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   |
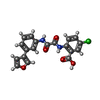
| #4: Chemical | ChemComp-RV2 / |
| #5: Water | ChemComp-HOH / |
| Has ligand of interest | Y |
| Has protein modification | Y |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.24 Å3/Da / Density % sol: 45 % / Mosaicity: 0.21 ° |
|---|---|
| Crystal grow | Temperature: 277 K / Method: vapor diffusion, hanging drop / pH: 6.5 / Details: 0.1 M BIS-TRIS, 10% w/v PEG 3350 |
-Data collection
| Diffraction | Mean temperature: 100 K / Serial crystal experiment: N | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  SOLEIL SOLEIL  / Beamline: PROXIMA 1 / Wavelength: 0.99187 Å / Beamline: PROXIMA 1 / Wavelength: 0.99187 Å | ||||||||||||||||||||||||||||||
| Detector | Type: DECTRIS PILATUS3 6M / Detector: PIXEL / Date: Oct 1, 2017 | ||||||||||||||||||||||||||||||
| Radiation | Monochromator: Si (111) monochromator / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray | ||||||||||||||||||||||||||||||
| Radiation wavelength | Wavelength: 0.99187 Å / Relative weight: 1 | ||||||||||||||||||||||||||||||
| Reflection | Resolution: 2.13→94.34 Å / Num. obs: 33767 / % possible obs: 99.6 % / Redundancy: 3.7 % / CC1/2: 0.997 / Rmerge(I) obs: 0.077 / Rpim(I) all: 0.046 / Rrim(I) all: 0.09 / Net I/σ(I): 8.1 / Num. measured all: 124847 / Scaling rejects: 1 | ||||||||||||||||||||||||||||||
| Reflection shell | Diffraction-ID: 1
|
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 6GWN Resolution: 2.27→43.8 Å / SU ML: 0.33 / Cross valid method: THROUGHOUT / σ(F): 1.37 / Phase error: 31.48 / Stereochemistry target values: ML
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å / Solvent model: FLAT BULK SOLVENT MODEL | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso max: 106.02 Å2 / Biso mean: 58.3645 Å2 / Biso min: 29.52 Å2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: final / Resolution: 2.27→43.8 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Refine-ID: X-RAY DIFFRACTION / Rfactor Rfree error: 0 / Total num. of bins used: 15
|
 Movie
Movie Controller
Controller












 PDBj
PDBj