+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 6yvt | ||||||
|---|---|---|---|---|---|---|---|
| Title | HIF prolyl hydroxylase 2 (PHD2/ EGLN1) in complex with MD-253 | ||||||
 Components Components | Egl nine homolog 1 | ||||||
 Keywords Keywords | OXIDOREDUCTASE / NON-HEME DIOXYGENASE / IRON / 2-OXOGLUTARATE / HYPOXIA-INDUCIBLE FACTOR / HIF / HIF PROLYL HYDROXYLASE DOMAIN 2 / PHD2 / EGLN1 / OXYGENASE / HYPOXIA / DNA-BINDING / METAL-BINDING / TRANSCRIPTION / HELIX-LOOP-HELIX-BETA / DSBH / FACIAL TRIAD / CYTOPLASM / TRANSCRIPTION/EPIGENETIC REGULATION / SIGNALING / DEVELOPMENT / CELL STRUCTURE / BETA-HYDROXYLATION / TRANSCRIPTION ACTIVATOR/INHIBITOR / UBL CONJUGATION / POLYMORPHISM / VITAMIN C / ZINC-FINGER / FAMILIAL ERYTHROCYTOSIS / BREAST CANCER / TRANSCRIPTION COMPLEX | ||||||
| Function / homology |  Function and homology information Function and homology informationpeptidyl-proline 4-dioxygenase activity / hypoxia-inducible factor-proline dioxygenase / hypoxia-inducible factor-proline dioxygenase activity / negative regulation of hypoxia-inducible factor-1alpha signaling pathway / peptidyl-proline dioxygenase activity / regulation protein catabolic process at postsynapse / intracellular oxygen homeostasis / regulation of modification of postsynaptic structure / labyrinthine layer development / 2-oxoglutarate-dependent dioxygenase activity ...peptidyl-proline 4-dioxygenase activity / hypoxia-inducible factor-proline dioxygenase / hypoxia-inducible factor-proline dioxygenase activity / negative regulation of hypoxia-inducible factor-1alpha signaling pathway / peptidyl-proline dioxygenase activity / regulation protein catabolic process at postsynapse / intracellular oxygen homeostasis / regulation of modification of postsynaptic structure / labyrinthine layer development / 2-oxoglutarate-dependent dioxygenase activity / heart trabecula formation / cardiac muscle tissue morphogenesis / L-ascorbic acid binding / response to nitric oxide / ventricular septum morphogenesis / regulation of angiogenesis / enzyme inhibitor activity / regulation of neuron apoptotic process / ferrous iron binding / Oxygen-dependent proline hydroxylation of Hypoxia-inducible Factor Alpha / cellular response to hypoxia / intracellular iron ion homeostasis / response to hypoxia / postsynaptic density / intracellular membrane-bounded organelle / glutamatergic synapse / enzyme binding / positive regulation of transcription by RNA polymerase II / zinc ion binding / nucleus / cytoplasm / cytosol Similarity search - Function | ||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  MOLECULAR REPLACEMENT / Resolution: 2.847 Å MOLECULAR REPLACEMENT / Resolution: 2.847 Å | ||||||
 Authors Authors | Chowdhury, R. / Demetriades, M. / Schofield, C.J. | ||||||
 Citation Citation |  Journal: Angew.Chem.Int.Ed.Engl. / Year: 2012 Journal: Angew.Chem.Int.Ed.Engl. / Year: 2012Title: Dynamic combinatorial chemistry employing boronic acids/boronate esters leads to potent oxygenase inhibitors. Authors: Demetriades, M. / Leung, I.K. / Chowdhury, R. / Chan, M.C. / McDonough, M.A. / Yeoh, K.K. / Tian, Y.M. / Claridge, T.D. / Ratcliffe, P.J. / Woon, E.C. / Schofield, C.J. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  6yvt.cif.gz 6yvt.cif.gz | 435.8 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb6yvt.ent.gz pdb6yvt.ent.gz | 359.3 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  6yvt.json.gz 6yvt.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/yv/6yvt https://data.pdbj.org/pub/pdb/validation_reports/yv/6yvt ftp://data.pdbj.org/pub/pdb/validation_reports/yv/6yvt ftp://data.pdbj.org/pub/pdb/validation_reports/yv/6yvt | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  2g19S S: Starting model for refinement |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| 2 | 
| ||||||||
| 3 | 
| ||||||||
| 4 | 
| ||||||||
| 5 | 
| ||||||||
| 6 | 
| ||||||||
| Unit cell |
|
- Components
Components
-Protein , 1 types, 6 molecules ABCDEF
| #1: Protein | Mass: 28096.941 Da / Num. of mol.: 6 Source method: isolated from a genetically manipulated source Details: CATALYTIC DOMAIN (RESIDUES 181-426) / Source: (gene. exp.)  Homo sapiens (human) / Gene: EGLN1, C1orf12, PNAS-118, PNAS-137 / Plasmid: PET28A(+) / Production host: Homo sapiens (human) / Gene: EGLN1, C1orf12, PNAS-118, PNAS-137 / Plasmid: PET28A(+) / Production host:  References: UniProt: Q9GZT9, hypoxia-inducible factor-proline dioxygenase |
|---|
-Non-polymers , 5 types, 71 molecules 
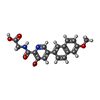







| #2: Chemical | ChemComp-MN / #3: Chemical | ChemComp-PW2 / #4: Chemical | ChemComp-GOL / | #5: Chemical | ChemComp-SO4 / | #6: Water | ChemComp-HOH / | |
|---|
-Details
| Has ligand of interest | Y |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 3.2 Å3/Da / Density % sol: 61.53 % |
|---|---|
| Crystal grow | Temperature: 293 K / Method: vapor diffusion, sitting drop / pH: 6.5 Details: Sample: 19.0 mg/ml PHD2 (50 mM Tris.HCl pH 7.5), 1.0 mM MnCl2 and 1.2 mM compound. Reservoir: 1.88 M ammonium sulfate, 0.1 M MES pH 6.5 and 5-7% dioxoane (v/v). Cryo-protection: 30% v/v glycerol. |
-Data collection
| Diffraction | Mean temperature: 100 K / Serial crystal experiment: N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diffraction source | Source:  ROTATING ANODE / Type: RIGAKU FR-E SUPERBRIGHT / Wavelength: 1.5418 Å ROTATING ANODE / Type: RIGAKU FR-E SUPERBRIGHT / Wavelength: 1.5418 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Detector | Type: RIGAKU SATURN 944 / Detector: CCD / Date: May 6, 2011 / Details: VARIMAX HF | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Radiation | Monochromator: CONFOCAL / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Radiation wavelength | Wavelength: 1.5418 Å / Relative weight: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reflection | Resolution: 2.847→50 Å / Num. obs: 40604 / % possible obs: 99.4 % / Redundancy: 5.3 % / Biso Wilson estimate: 48.4 Å2 / CC1/2: 0.998 / Rmerge(I) obs: 0.18 / Rpim(I) all: 0.112 / Rrim(I) all: 0.2 / Χ2: 1.098 / Net I/σ(I): 6.71 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reflection shell | Diffraction-ID: 1
|
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 2G19 Resolution: 2.847→23.753 Å / SU ML: 0.44 / Cross valid method: THROUGHOUT / σ(F): 1.96 / Phase error: 27.14 / Stereochemistry target values: ML
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å / Solvent model: FLAT MODEL / Bsol: 57.9 Å2 / ksol: 0.45 e/Å3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso max: 211.06 Å2 / Biso mean: 55.0728 Å2 / Biso min: 19.96 Å2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: final / Resolution: 2.847→23.753 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Refine-ID: X-RAY DIFFRACTION / Rfactor Rfree error: 0
|
 Movie
Movie Controller
Controller
















 PDBj
PDBj




