+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 6yie | ||||||
|---|---|---|---|---|---|---|---|
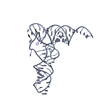
| Title | Structure of a Borealin-INCENP-Survivin complex | ||||||
 Components Components |
| ||||||
 Keywords Keywords | CELL CYCLE | ||||||
| Function / homology |  Function and homology information Function and homology informationcentral element / meiotic spindle midzone / survivin complex / meiotic spindle midzone assembly / : / positive regulation of mitotic sister chromatid separation / positive regulation of mitotic cytokinesis / metaphase chromosome alignment / positive regulation of mitotic cell cycle spindle assembly checkpoint / positive regulation of exit from mitosis ...central element / meiotic spindle midzone / survivin complex / meiotic spindle midzone assembly / : / positive regulation of mitotic sister chromatid separation / positive regulation of mitotic cytokinesis / metaphase chromosome alignment / positive regulation of mitotic cell cycle spindle assembly checkpoint / positive regulation of exit from mitosis / mitotic spindle midzone assembly / positive regulation of attachment of mitotic spindle microtubules to kinetochore / chromocenter / interphase microtubule organizing center / chromosome passenger complex / lateral element / protein-containing complex localization / cysteine-type endopeptidase inhibitor activity involved in apoptotic process / cobalt ion binding / mitotic metaphase chromosome alignment / nuclear chromosome / mitotic spindle assembly checkpoint signaling / spindle midzone / TP53 regulates transcription of several additional cell death genes whose specific roles in p53-dependent apoptosis remain uncertain / mitotic cytokinesis / mitotic sister chromatid segregation / SUMOylation of DNA replication proteins / chromosome, centromeric region / mitotic spindle assembly / cysteine-type endopeptidase inhibitor activity / chromosome organization / pericentric heterochromatin / intercellular bridge / cytoplasmic microtubule / Amplification of signal from unattached kinetochores via a MAD2 inhibitory signal / centriole / Mitotic Prometaphase / positive regulation of mitotic cell cycle / EML4 and NUDC in mitotic spindle formation / tubulin binding / molecular function activator activity / Resolution of Sister Chromatid Cohesion / mitotic spindle organization / spindle microtubule / chromosome segregation / sensory perception of sound / RHO GTPases Activate Formins / kinetochore / small GTPase binding / G2/M transition of mitotic cell cycle / spindle / Separation of Sister Chromatids / mitotic cell cycle / protein-folding chaperone binding / microtubule cytoskeleton / Neddylation / Interleukin-4 and Interleukin-13 signaling / midbody / microtubule binding / microtubule / nuclear body / protein heterodimerization activity / cell division / negative regulation of DNA-templated transcription / positive regulation of cell population proliferation / apoptotic process / negative regulation of apoptotic process / nucleolus / enzyme binding / protein homodimerization activity / protein-containing complex / zinc ion binding / nucleoplasm / metal ion binding / identical protein binding / nucleus / cytosol / cytoplasm Similarity search - Function | ||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 3.49 Å MOLECULAR REPLACEMENT / Resolution: 3.49 Å | ||||||
 Authors Authors | Serena, M. / Elliott, P.R. / Barr, F.A. | ||||||
| Funding support |  United Kingdom, 1items United Kingdom, 1items
| ||||||
 Citation Citation |  Journal: J.Cell Biol. / Year: 2020 Journal: J.Cell Biol. / Year: 2020Title: Molecular basis of MKLP2-dependent Aurora B transport from chromatin to the anaphase central spindle. Authors: Serena, M. / Bastos, R.N. / Elliott, P.R. / Barr, F.A. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  6yie.cif.gz 6yie.cif.gz | 318.6 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb6yie.ent.gz pdb6yie.ent.gz | 219.2 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  6yie.json.gz 6yie.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/yi/6yie https://data.pdbj.org/pub/pdb/validation_reports/yi/6yie ftp://data.pdbj.org/pub/pdb/validation_reports/yi/6yie ftp://data.pdbj.org/pub/pdb/validation_reports/yi/6yie | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  6yifC  6yihC  6yipC  2qfaS S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2 | 
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Unit cell |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Noncrystallographic symmetry (NCS) | NCS domain:
NCS domain segments:
NCS ensembles :
|
- Components
Components
| #1: Protein | Mass: 16568.902 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: BIRC5, API4, IAP4 / Plasmid: pETDuet1 / Production host: Homo sapiens (human) / Gene: BIRC5, API4, IAP4 / Plasmid: pETDuet1 / Production host:  #2: Protein | Mass: 11617.326 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: CDCA8, PESCRG3 / Production host: Homo sapiens (human) / Gene: CDCA8, PESCRG3 / Production host:  #3: Protein | Mass: 7018.110 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: INCENP / Plasmid: PFAT2-His6-GST / Production host: Homo sapiens (human) / Gene: INCENP / Plasmid: PFAT2-His6-GST / Production host:  #4: Chemical | Has ligand of interest | N | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.09 Å3/Da / Density % sol: 41.11 % |
|---|---|
| Crystal grow | Temperature: 298 K / Method: vapor diffusion / pH: 6 / Details: 5 % (w/v) PEG 3,350, 50 mM MES pH 6.0 |
-Data collection
| Diffraction | Mean temperature: 110 K / Serial crystal experiment: N |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  PETRA III, EMBL c/o DESY PETRA III, EMBL c/o DESY  / Beamline: P14 (MX2) / Wavelength: 0.9763 Å / Beamline: P14 (MX2) / Wavelength: 0.9763 Å |
| Detector | Type: DECTRIS EIGER X 16M / Detector: PIXEL / Date: Jun 21, 2017 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.9763 Å / Relative weight: 1 |
| Reflection | Resolution: 3.49→78.82 Å / Num. obs: 6947 / % possible obs: 88.5 % / Redundancy: 3.7 % / Biso Wilson estimate: 65.27 Å2 / CC1/2: 0.992 / Rmerge(I) obs: 0.162 / Net I/σ(I): 3.5 |
| Reflection shell | Resolution: 3.49→3.83 Å / Rmerge(I) obs: 0.435 / Mean I/σ(I) obs: 1.7 / Num. unique obs: 1644 / CC1/2: 0.902 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 2QFA Resolution: 3.49→66.7 Å / SU ML: 0.4279 / Cross valid method: FREE R-VALUE / σ(F): 1.36 / Phase error: 35.3698
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 64.38 Å2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 3.49→66.7 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Refine-ID: X-RAY DIFFRACTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group |
|
 Movie
Movie Controller
Controller












 PDBj
PDBj














