[English] 日本語
 Yorodumi
Yorodumi- PDB-6wi8: Inhibitor compound-induced confrontational change in Ring1b-Bmi1 ... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 6wi8 | ||||||
|---|---|---|---|---|---|---|---|
| Title | Inhibitor compound-induced confrontational change in Ring1b-Bmi1 domain structure | ||||||
 Components Components | E3 ubiquitin-protein ligase RING2,Polycomb complex protein BMI-1 chimera | ||||||
 Keywords Keywords | LIGASE / E3 ubiquitin ligase Ring1b / Bmi1 | ||||||
| Function / homology |  Function and homology information Function and homology informationPRC1 complex / regulation of kidney development / RING-like zinc finger domain binding / segment specification / rostrocaudal neural tube patterning / ubiquitin-protein transferase activator activity / regulation of adaxial/abaxial pattern formation / embryonic skeletal system morphogenesis / somatic stem cell division / positive regulation of immature T cell proliferation in thymus ...PRC1 complex / regulation of kidney development / RING-like zinc finger domain binding / segment specification / rostrocaudal neural tube patterning / ubiquitin-protein transferase activator activity / regulation of adaxial/abaxial pattern formation / embryonic skeletal system morphogenesis / somatic stem cell division / positive regulation of immature T cell proliferation in thymus / PcG protein complex / SUMOylation of DNA methylation proteins / SUMOylation of RNA binding proteins / positive regulation of ubiquitin-protein transferase activity / DNA methylation-dependent constitutive heterochromatin formation / negative regulation of gene expression, epigenetic / Transcriptional Regulation by E2F6 / RUNX1 interacts with co-factors whose precise effect on RUNX1 targets is not known / humoral immune response / hemopoiesis / negative regulation of apoptotic signaling pathway / cellular response to dexamethasone stimulus / cellular response to interleukin-1 / ubiquitin ligase complex / SUMOylation of DNA damage response and repair proteins / positive regulation of B cell proliferation / heterochromatin / SUMOylation of transcription cofactors / SUMOylation of chromatin organization proteins / Regulation of PTEN gene transcription / apoptotic signaling pathway / promoter-specific chromatin binding / brain development / RING-type E3 ubiquitin transferase / positive regulation of fibroblast proliferation / ubiquitin protein ligase activity / regulation of gene expression / Oxidative Stress Induced Senescence / in utero embryonic development / nuclear body / protein ubiquitination / chromatin remodeling / negative regulation of transcription by RNA polymerase II / zinc ion binding / nucleoplasm / nucleus / cytosol Similarity search - Function | ||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 3.092 Å MOLECULAR REPLACEMENT / Resolution: 3.092 Å | ||||||
 Authors Authors | Cho, H.J. / Cierpicki, T. | ||||||
 Citation Citation |  Journal: Nat.Chem.Biol. / Year: 2021 Journal: Nat.Chem.Biol. / Year: 2021Title: Small-molecule inhibitors targeting Polycomb repressive complex 1 RING domain. Authors: Shukla, S. / Ying, W. / Gray, F. / Yao, Y. / Simes, M.L. / Zhao, Q. / Miao, H. / Cho, H.J. / Gonzalez-Alonso, P. / Winkler, A. / Lund, G. / Purohit, T. / Kim, E. / Zhang, X. / Ray, J.M. / ...Authors: Shukla, S. / Ying, W. / Gray, F. / Yao, Y. / Simes, M.L. / Zhao, Q. / Miao, H. / Cho, H.J. / Gonzalez-Alonso, P. / Winkler, A. / Lund, G. / Purohit, T. / Kim, E. / Zhang, X. / Ray, J.M. / He, S. / Nikolaidis, C. / Ndoj, J. / Wang, J. / Jaremko, L. / Jaremko, M. / Ryan, R.J.H. / Guzman, M.L. / Grembecka, J. / Cierpicki, T. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  6wi8.cif.gz 6wi8.cif.gz | 177.7 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb6wi8.ent.gz pdb6wi8.ent.gz | 139.6 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  6wi8.json.gz 6wi8.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/wi/6wi8 https://data.pdbj.org/pub/pdb/validation_reports/wi/6wi8 ftp://data.pdbj.org/pub/pdb/validation_reports/wi/6wi8 ftp://data.pdbj.org/pub/pdb/validation_reports/wi/6wi8 | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  6wi7SC  7nd1C S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 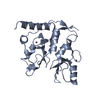
| ||||||||
| 2 | 
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 24322.715 Da / Num. of mol.: 2 Fragment: RING-2 (UNP residues 10-116) + BMI-1 (UNP residues 1-104) Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: RNF2, BMI1, PCGF4, RNF51 / Plasmid: pET32a / Production host: Homo sapiens (human) / Gene: RNF2, BMI1, PCGF4, RNF51 / Plasmid: pET32a / Production host:  #2: Chemical | ChemComp-ZN / #3: Water | ChemComp-HOH / | Has ligand of interest | Y | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.26 Å3/Da / Density % sol: 45.57 % |
|---|---|
| Crystal grow | Temperature: 277.15 K / Method: vapor diffusion, sitting drop / pH: 4.5 Details: 100 mM acetate, pH 4.5, 200 mM lithium sulfate, 2.5 M sodium chloride |
-Data collection
| Diffraction | Mean temperature: 110 K / Serial crystal experiment: N |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  APS APS  / Beamline: 21-ID-F / Wavelength: 0.978 Å / Beamline: 21-ID-F / Wavelength: 0.978 Å |
| Detector | Type: MARMOSAIC 225 mm CCD / Detector: CCD / Date: Dec 11, 2014 / Details: NULL |
| Radiation | Monochromator: diamond(111) / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.978 Å / Relative weight: 1 |
| Reflection | Resolution: 3.09→50 Å / Num. obs: 7292 / % possible obs: 98.5 % / Redundancy: 3.7 % / Rsym value: 0.11 / Net I/σ(I): 13.28 |
| Reflection shell | Resolution: 3.1→3.15 Å / Num. unique obs: 7320 / Rsym value: 0.443 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: PDB entry 6WI7 Resolution: 3.092→41.721 Å / SU ML: 0.43 / Cross valid method: THROUGHOUT / σ(F): 1.38 / Phase error: 27.15
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso max: 194.19 Å2 / Biso mean: 65.5982 Å2 / Biso min: 17.04 Å2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: final / Resolution: 3.092→41.721 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Refine-ID: X-RAY DIFFRACTION / Rfactor Rfree error: 0
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Refine-ID: X-RAY DIFFRACTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group |
|
 Movie
Movie Controller
Controller












 PDBj
PDBj













