[English] 日本語
 Yorodumi
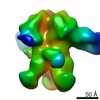
Yorodumi- PDB-6ppb: Kaposi's sarcoma-associated herpesvirus (KSHV), C5 portal vertex ... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 6ppb | |||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Kaposi's sarcoma-associated herpesvirus (KSHV), C5 portal vertex structure | |||||||||||||||||||||||||||||||||||||||||||||
 Components Components |
| |||||||||||||||||||||||||||||||||||||||||||||
 Keywords Keywords | VIRAL PROTEIN / genome / portal / capsid / genome packaging / VIRUS | |||||||||||||||||||||||||||||||||||||||||||||
| Function / homology |  Function and homology information Function and homology informationicosahedral viral capsid / T=16 icosahedral viral capsid / viral genome packaging / viral tegument / viral capsid assembly / viral process / chromosome organization / viral penetration into host nucleus / viral capsid / host cell ...icosahedral viral capsid / T=16 icosahedral viral capsid / viral genome packaging / viral tegument / viral capsid assembly / viral process / chromosome organization / viral penetration into host nucleus / viral capsid / host cell / symbiont-mediated perturbation of host ubiquitin-like protein modification / host cell cytoplasm / ubiquitinyl hydrolase 1 / cysteine-type deubiquitinase activity / Hydrolases; Acting on peptide bonds (peptidases); Cysteine endopeptidases / viral envelope / symbiont entry into host cell / host cell nucleus / structural molecule activity / proteolysis / DNA binding Similarity search - Function | |||||||||||||||||||||||||||||||||||||||||||||
| Biological species |   Human herpesvirus 8 Human herpesvirus 8 | |||||||||||||||||||||||||||||||||||||||||||||
| Method | ELECTRON MICROSCOPY / single particle reconstruction / cryo EM / Resolution: 4.3 Å | |||||||||||||||||||||||||||||||||||||||||||||
 Authors Authors | Gong, D. / Dai, X. / Jih, J. / Liu, Y.T. / Bi, G.Q. / Sun, R. / Zhou, Z.H. | |||||||||||||||||||||||||||||||||||||||||||||
| Funding support |  United States, United States,  China, 14items China, 14items
| |||||||||||||||||||||||||||||||||||||||||||||
 Citation Citation |  Journal: Cell / Year: 2019 Journal: Cell / Year: 2019Title: DNA-Packing Portal and Capsid-Associated Tegument Complexes in the Tumor Herpesvirus KSHV. Authors: Danyang Gong / Xinghong Dai / Jonathan Jih / Yun-Tao Liu / Guo-Qiang Bi / Ren Sun / Z Hong Zhou /   Abstract: Assembly of Kaposi's sarcoma-associated herpesvirus (KSHV) begins at a bacteriophage-like portal complex that nucleates formation of an icosahedral capsid with capsid-associated tegument complexes ...Assembly of Kaposi's sarcoma-associated herpesvirus (KSHV) begins at a bacteriophage-like portal complex that nucleates formation of an icosahedral capsid with capsid-associated tegument complexes (CATCs) and facilitates translocation of an ∼150-kb dsDNA genome, followed by acquisition of a pleomorphic tegument and envelope. Because of deviation from icosahedral symmetry, KSHV portal and tegument structures have largely been obscured in previous studies. Using symmetry-relaxed cryo-EM, we determined the in situ structure of the KSHV portal and its interactions with surrounding capsid proteins, CATCs, and the terminal end of KSHV's dsDNA genome. Our atomic models of the portal and capsid/CATC, together with visualization of CATCs' variable occupancy and alternate orientation of CATC-interacting vertex triplexes, suggest a mechanism whereby the portal orchestrates procapsid formation and asymmetric long-range determination of CATC attachment during DNA packaging prior to pleomorphic tegumentation/envelopment. Structure-based mutageneses confirm that a triplex deep binding groove for CATCs is a hotspot that holds promise for antiviral development. | |||||||||||||||||||||||||||||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  6ppb.cif.gz 6ppb.cif.gz | 1.5 MB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb6ppb.ent.gz pdb6ppb.ent.gz | 1.1 MB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  6ppb.json.gz 6ppb.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/pp/6ppb https://data.pdbj.org/pub/pdb/validation_reports/pp/6ppb ftp://data.pdbj.org/pub/pdb/validation_reports/pp/6ppb ftp://data.pdbj.org/pub/pdb/validation_reports/pp/6ppb | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  20432MC  6ppdC  6pphC  6ppiC C: citing same article ( M: map data used to model this data |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
-Capsid vertex component ... , 2 types, 3 molecules klm
| #1: Protein | Mass: 49586.555 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)   Human herpesvirus 8 / Strain: GK18 / References: UniProt: Q76RH8, UniProt: F5HB39*PLUS Human herpesvirus 8 / Strain: GK18 / References: UniProt: Q76RH8, UniProt: F5HB39*PLUS |
|---|---|
| #2: Protein | Mass: 61494.383 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Source: (natural)   Human herpesvirus 8 / Strain: GK18 / References: UniProt: Q76RI7, UniProt: Q2HRB3*PLUS Human herpesvirus 8 / Strain: GK18 / References: UniProt: Q76RI7, UniProt: Q2HRB3*PLUS |
-Protein , 3 types, 10 molecules noSTWX0123
| #3: Protein | Mass: 290024.844 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Source: (natural)   Human herpesvirus 8 / Strain: GK18 Human herpesvirus 8 / Strain: GK18References: UniProt: Q2HR64, ubiquitinyl hydrolase 1, Hydrolases; Acting on peptide bonds (peptidases); Cysteine endopeptidases #4: Protein | Mass: 153574.188 Da / Num. of mol.: 4 / Source method: isolated from a natural source / Source: (natural)   Human herpesvirus 8 / Strain: GK18 / References: UniProt: Q2HRA7 Human herpesvirus 8 / Strain: GK18 / References: UniProt: Q2HRA7#7: Protein | Mass: 18597.824 Da / Num. of mol.: 4 / Source method: isolated from a natural source / Source: (natural)   Human herpesvirus 8 / Strain: GK18 / References: UniProt: Q76RF4, UniProt: Q2HR63*PLUS Human herpesvirus 8 / Strain: GK18 / References: UniProt: Q76RF4, UniProt: Q2HR63*PLUS |
|---|
-Triplex capsid protein ... , 2 types, 6 molecules b5cd67
| #5: Protein | Mass: 36374.840 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Source: (natural)   Human herpesvirus 8 / Strain: GK18 / References: UniProt: Q76RF6, UniProt: F5H8Y5*PLUS Human herpesvirus 8 / Strain: GK18 / References: UniProt: Q76RF6, UniProt: F5H8Y5*PLUS#6: Protein | Mass: 34278.473 Da / Num. of mol.: 4 / Source method: isolated from a natural source / Source: (natural)   Human herpesvirus 8 / Strain: GK18 / References: UniProt: Q98832, UniProt: F5HGN8*PLUS Human herpesvirus 8 / Strain: GK18 / References: UniProt: Q98832, UniProt: F5HGN8*PLUS |
|---|
-Details
| Has protein modification | Y |
|---|
-Experimental details
-Experiment
| Experiment | Method: ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method: single particle reconstruction |
- Sample preparation
Sample preparation
| Component | Name: Human gammaherpesvirus 8 / Type: VIRUS / Entity ID: all / Source: NATURAL |
|---|---|
| Molecular weight | Experimental value: NO |
| Source (natural) | Organism:   Human gammaherpesvirus 8 / Strain: BAC16 Human gammaherpesvirus 8 / Strain: BAC16 |
| Details of virus | Empty: NO / Enveloped: YES / Isolate: STRAIN / Type: VIRION |
| Natural host | Organism: Homo sapiens |
| Virus shell | Name: Capsid / Diameter: 1250 nm / Triangulation number (T number): 16 |
| Buffer solution | pH: 7.4 |
| Specimen | Embedding applied: NO / Shadowing applied: NO / Staining applied: NO / Vitrification applied: YES |
| Vitrification | Instrument: HOMEMADE PLUNGER / Cryogen name: ETHANE / Chamber temperature: 298 K Details: The sample was manually blotted and frozen with a homemade plunger. |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: FEI TITAN KRIOS |
| Electron gun | Electron source:  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD / Nominal magnification: 14000 X / Calibrated magnification: 24271 X / Cs: 2.7 mm |
| Specimen holder | Cryogen: NITROGEN / Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Temperature (max): 79 K |
| Image recording | Average exposure time: 13 sec. / Electron dose: 25 e/Å2 / Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Num. of real images: 8007 |
| Image scans | Sampling size: 2.5 µm / Width: 1440 / Height: 1440 / Movie frames/image: 26 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EM software |
| ||||||||||||||||||||||||||||||||||||||||||||
| CTF correction | Type: PHASE FLIPPING AND AMPLITUDE CORRECTION | ||||||||||||||||||||||||||||||||||||||||||||
| Particle selection | Num. of particles selected: 44328 | ||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Point symmetry: C5 (5 fold cyclic) | ||||||||||||||||||||||||||||||||||||||||||||
| 3D reconstruction | Resolution: 4.3 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 39773 / Algorithm: FOURIER SPACE / Symmetry type: POINT | ||||||||||||||||||||||||||||||||||||||||||||
| Atomic model building | B value: 177.4 / Protocol: AB INITIO MODEL / Space: REAL / Target criteria: Correlation coefficient | ||||||||||||||||||||||||||||||||||||||||||||
| Refinement | Stereochemistry target values: GeoStd + Monomer Library | ||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller














 PDBj
PDBj
