| Entry | Database: PDB / ID: 6p3d
|
|---|
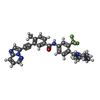
| Title | The co-crystal structure of BRAF(V600E) with ponatinib |
|---|
 Components Components | Serine/threonine-protein kinase B-raf |
|---|
 Keywords Keywords | TRANSFERASE/TRANSFERASE INHIBITOR / kinase / inhibitor / cancer / melanoma / TRANSFERASE-TRANSFERASE INHIBITOR complex / ONCOPROTEIN |
|---|
| Function / homology |  Function and homology information Function and homology information
CD4-positive, alpha-beta T cell differentiation / positive regulation of axon regeneration / CD4-positive or CD8-positive, alpha-beta T cell lineage commitment / negative regulation of synaptic vesicle exocytosis / Signalling to p38 via RIT and RIN / head morphogenesis / ARMS-mediated activation / endothelial cell apoptotic process / myeloid progenitor cell differentiation / SHOC2 M1731 mutant abolishes MRAS complex function ...CD4-positive, alpha-beta T cell differentiation / positive regulation of axon regeneration / CD4-positive or CD8-positive, alpha-beta T cell lineage commitment / negative regulation of synaptic vesicle exocytosis / Signalling to p38 via RIT and RIN / head morphogenesis / ARMS-mediated activation / endothelial cell apoptotic process / myeloid progenitor cell differentiation / SHOC2 M1731 mutant abolishes MRAS complex function / Gain-of-function MRAS complexes activate RAF signaling / negative regulation of fibroblast migration / positive regulation of D-glucose transmembrane transport / establishment of protein localization to membrane / regulation of T cell differentiation / positive regulation of axonogenesis / Negative feedback regulation of MAPK pathway / Frs2-mediated activation / stress fiber assembly / face development / MAP kinase kinase activity / thyroid gland development / synaptic vesicle exocytosis / somatic stem cell population maintenance / positive regulation of peptidyl-serine phosphorylation / MAP kinase kinase kinase activity / negative regulation of endothelial cell apoptotic process / postsynaptic modulation of chemical synaptic transmission / positive regulation of stress fiber assembly / sperm end piece / ERK1 and ERK2 cascade / positive regulation of substrate adhesion-dependent cell spreading / substrate adhesion-dependent cell spreading / cellular response to calcium ion / sperm principal piece / thymus development / animal organ morphogenesis / RAF activation / Spry regulation of FGF signaling / Signaling by high-kinase activity BRAF mutants / MAP2K and MAPK activation / visual learning / cellular response to xenobiotic stimulus / epidermal growth factor receptor signaling pathway / centriolar satellite / Negative regulation of MAPK pathway / Signaling by RAF1 mutants / Signaling by moderate kinase activity BRAF mutants / Paradoxical activation of RAF signaling by kinase inactive BRAF / Signaling downstream of RAS mutants / Signaling by BRAF and RAF1 fusions / long-term synaptic potentiation / T cell differentiation in thymus / sperm midpiece / MAPK cascade / T cell receptor signaling pathway / regulation of cell population proliferation / presynapse / cell body / scaffold protein binding / negative regulation of neuron apoptotic process / protein phosphorylation / protein kinase activity / positive regulation of ERK1 and ERK2 cascade / non-specific serine/threonine protein kinase / neuron projection / postsynapse / cilium / ciliary basal body / protein serine kinase activity / protein serine/threonine kinase activity / calcium ion binding / positive regulation of gene expression / negative regulation of apoptotic process / glutamatergic synapse / mitochondrion / zinc ion binding / ATP binding / identical protein binding / nucleus / plasma membrane / cytoplasm / cytosolSimilarity search - Function Raf-like Ras-binding domain / Raf-like Ras-binding / Ras-binding domain (RBD) profile. / Raf-like Ras-binding domain / Diacylglycerol/phorbol-ester binding / : / Phorbol esters/diacylglycerol binding domain (C1 domain) / Zinc finger phorbol-ester/DAG-type signature. / Zinc finger phorbol-ester/DAG-type profile. / Protein kinase C conserved region 1 (C1) domains (Cysteine-rich domains) ...Raf-like Ras-binding domain / Raf-like Ras-binding / Ras-binding domain (RBD) profile. / Raf-like Ras-binding domain / Diacylglycerol/phorbol-ester binding / : / Phorbol esters/diacylglycerol binding domain (C1 domain) / Zinc finger phorbol-ester/DAG-type signature. / Zinc finger phorbol-ester/DAG-type profile. / Protein kinase C conserved region 1 (C1) domains (Cysteine-rich domains) / Protein kinase C-like, phorbol ester/diacylglycerol-binding domain / C1-like domain superfamily / Serine-threonine/tyrosine-protein kinase, catalytic domain / Protein tyrosine and serine/threonine kinase / Ubiquitin-like domain superfamily / Transferase(Phosphotransferase) domain 1 / Transferase(Phosphotransferase); domain 1 / Serine/threonine-protein kinase, active site / Serine/Threonine protein kinases active-site signature. / Serine/Threonine protein kinases, catalytic domain / Protein kinase, ATP binding site / Protein kinases ATP-binding region signature. / Protein kinase domain profile. / Protein kinase domain / Protein kinase-like domain superfamily / Orthogonal Bundle / Mainly AlphaSimilarity search - Domain/homology |
|---|
| Biological species |  Homo sapiens (human) Homo sapiens (human) |
|---|
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.11 Å MOLECULAR REPLACEMENT / Resolution: 2.11 Å |
|---|
 Authors Authors | Agianian, B. / Gavathiotis, E. |
|---|
| Funding support |  United States, 1items United States, 1items | Organization | Grant number | Country |
|---|
| National Institutes of Health/National Cancer Institute (NIH/NCI) | 1R01CA178394 |  United States United States |
|
|---|
 Citation Citation |  Journal: Nat Commun / Year: 2020Title Journal: Nat Commun / Year: 2020Title: Inhibitors of BRAF dimers using an allosteric site. Authors: Cotto-Rios, X.M. / Agianian, B. / Gitego, N. / Zacharioudakis, E. / Giricz, O. / Wu, Y. / Zou, Y. / Verma, A. / Poulikakos, P.I. / Gavathiotis, E.#1: Journal: Cancer Cell / Year: 2016Title: An Integrated Model of RAF Inhibitor Action Predicts Inhibitor Activity against Oncogenic BRAF Signaling. Authors: Karoulia, Z. / Wu, Y. / Ahmed, T.A. / Xin, Q. / Bollard, J. / Krepler, C. / Wu, X. / Zhang, C. / Bollag, G. / Herlyn, M. / Fagin, J.A. / Lujambio, A. / Gavathiotis, E. / Poulikakos, P.I. |
|---|
| History | | Deposition | May 23, 2019 | Deposition site: RCSB / Processing site: RCSB |
|---|
| Revision 1.0 | Sep 23, 2020 | Provider: repository / Type: Initial release |
|---|
| Revision 1.1 | Mar 13, 2024 | Group: Data collection / Database references / Category: chem_comp_atom / chem_comp_bond / database_2
Item: _database_2.pdbx_DOI / _database_2.pdbx_database_accession |
|---|
|
|---|
 Open data
Open data Basic information
Basic information Components
Components Keywords
Keywords Function and homology information
Function and homology information Homo sapiens (human)
Homo sapiens (human) X-RAY DIFFRACTION /
X-RAY DIFFRACTION /  SYNCHROTRON /
SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.11 Å
MOLECULAR REPLACEMENT / Resolution: 2.11 Å  Authors
Authors United States, 1items
United States, 1items  Citation
Citation Journal: Nat Commun / Year: 2020
Journal: Nat Commun / Year: 2020 Structure visualization
Structure visualization Molmil
Molmil Jmol/JSmol
Jmol/JSmol Downloads & links
Downloads & links Download
Download 6p3d.cif.gz
6p3d.cif.gz PDBx/mmCIF format
PDBx/mmCIF format pdb6p3d.ent.gz
pdb6p3d.ent.gz PDB format
PDB format 6p3d.json.gz
6p3d.json.gz PDBx/mmJSON format
PDBx/mmJSON format Other downloads
Other downloads https://data.pdbj.org/pub/pdb/validation_reports/p3/6p3d
https://data.pdbj.org/pub/pdb/validation_reports/p3/6p3d ftp://data.pdbj.org/pub/pdb/validation_reports/p3/6p3d
ftp://data.pdbj.org/pub/pdb/validation_reports/p3/6p3d Links
Links Assembly
Assembly

 Components
Components Homo sapiens (human) / Gene: BRAF, BRAF1, RAFB1 / Plasmid: MODIFIED PET28 / Production host:
Homo sapiens (human) / Gene: BRAF, BRAF1, RAFB1 / Plasmid: MODIFIED PET28 / Production host: 







 X-RAY DIFFRACTION / Number of used crystals: 1
X-RAY DIFFRACTION / Number of used crystals: 1  Sample preparation
Sample preparation SYNCHROTRON / Site:
SYNCHROTRON / Site:  APS
APS  / Beamline: 23-ID-D / Wavelength: 1.03324 Å
/ Beamline: 23-ID-D / Wavelength: 1.03324 Å Processing
Processing MOLECULAR REPLACEMENT / Resolution: 2.11→84.03 Å / Cor.coef. Fo:Fc: 0.951 / Cor.coef. Fo:Fc free: 0.919 / SU B: 4.571 / SU ML: 0.12 / Cross valid method: THROUGHOUT / σ(F): 0 / ESU R: 0.177 / ESU R Free: 0.175 / Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING
MOLECULAR REPLACEMENT / Resolution: 2.11→84.03 Å / Cor.coef. Fo:Fc: 0.951 / Cor.coef. Fo:Fc free: 0.919 / SU B: 4.571 / SU ML: 0.12 / Cross valid method: THROUGHOUT / σ(F): 0 / ESU R: 0.177 / ESU R Free: 0.175 / Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING Movie
Movie Controller
Controller














 PDBj
PDBj









